Visualizing Enzymes in Action
08/18/2020
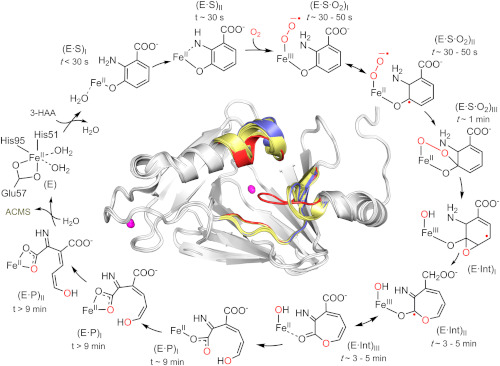
The catalytic reaction cycle of an enzyme critical to NAD+ biosynthesis. Time-resolved in crystallo reactions of the enzyme 3-Hydroxyanthranilate-3,4-dioxygenase (HAO) were revealed by UV-vis microspectroscopy, electron paramagnetic resonance (EPR) spectroscopy, and X-ray crystallography. The figure shows characterized reaction intermediates along with time windows for observing them in crystallo, offering a near-complete view of the complex HAO catalytic cycle.
[Reprinted under a Creative Commons license (CC BY 4.0) from Wang et al. 2020. DOI:10.1073/pnas.2005327117]
The enzyme 3-Hydroxyanthranilate-3,4-dioxygenase (HAO) is critical for biosynthesis of the coenzyme nicotinamide adenine dinucleotide (NAD+), which in turn is required for biosynthesis in all kingdoms of life. Although the role of this enzyme has long been known, its mechanism and regulation have remained a mystery. HAO is difficult to study because it creates unstable intermediates that can easily form cyclic by-products.
Scientists from the University of Texas at San Antonio and the University of Pennsylvania, Philadelphia, have determined the mechanism of the HAO enzyme by studying its crystalline state in which the reaction rate is slowed to minutes. They combined single-crystal UV-Vis spectroscopy and X-ray crystallography to probe changes in the active site and the overall conformation of HAO. The enzyme was first crystallized and then incubated with its aromatic substrate under anaerobic conditions. Subsequently, crystals of enzyme-substrate complex were exposed to oxygen for different lengths of time to interrogate any possible intermediates. The accumulation of catalytic intermediates at various timepoints were identified using the integrated single-crystal UV-Vis spectroscopy system at the Stanford Synchrotron Radiation Lightsource beamline 9-2 and X-ray diffraction data were collected for each corresponding timepoint.
The structural analysis provided a step-by-step visualization of the HAO cycle, revealing the intermediates involved and how quinolinic acid production is fine-tuned by the enzyme.
Related Links
References
Wang, Y., et al. 2020. “Observing 3-hydroxyanthranilate-3,4-dioxygenase in Action Through a Crystalline Lens,” Proceedings of the National Academy of Sciences USA 117(33), 19720–730. [DOI:10.1073/pnas.2005327117]
