3D Protein Structure Aids Search for Vaccine
11/16/2017
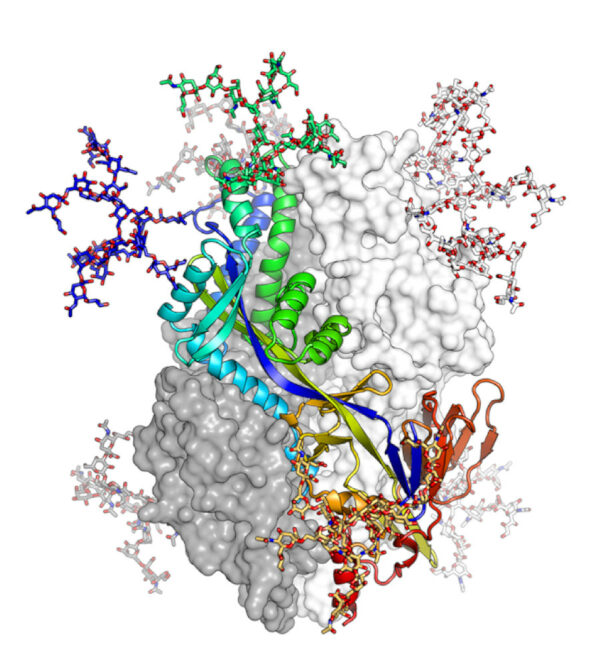
Scientists combined cryogenic macromolecular X-ray crystallography with modeling based on the known structure of a homologous molecule to reveal the 3D structure of the human metapneumovirus (hMPV) F glycoprotein, which mediates cell-virus membrane fusion and could be a potential target for an anti-viral vaccine. The three protomers of the pre-fusion F glycoprotein are shown as a rainbow ribbon, a white surface, and a gray surface. N-linked glycans observed in the structure are shown as sticks. [Reprinted under a Creative Commons Attribution 4.0 International (CC BY 4.0) license from Battles, M., et al. 2017. “Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein,” Nat Commun 8, 1528. DOI:10.1038/s41467-017-01708-9]
The Summary
Human metapneumovirus (hMPV) is a frequent cause of bronchitis in young children, but vaccine development has been hindered by an inability to produce the hMPV F glycoprotein, which mediates virus–cell membrane fusion and is the primary target of neutralizing antibodies.
Using cryogenic macromolecular crystallography and modeling based on the known structure of a homologous molecule, scientists revealed the 3D structure of the hMPV F glycoprotein.
This new knowledge about the viral structure should facilitate development of effective hMPV vaccine candidates.
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: 3D protein structure helps in the search for a vaccine
References
M. Battles, V. Más, E. Olmedillas, O. Cano, M. Vázquez, L. Rodríguez, J. Melero, & J. McLellan. “Structure and immunogenicity of pre-fusion-stabilized human metapneumovirus F glycoprotein,” Nat Commun 8, 1528 (2017). DOI: [10.1038/s41467-017-01708-9]
