An Efficient and Economical Strategy for the Design of Functional Metalloproteins
02/18/2019
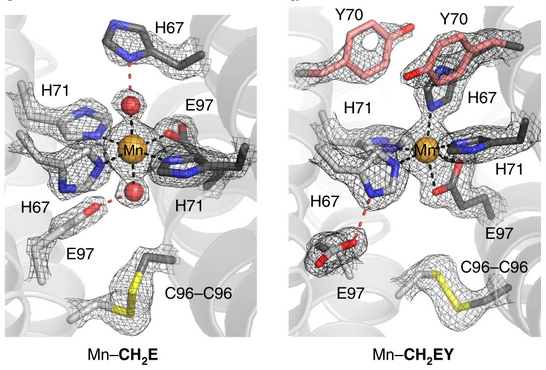
Close-up view of the manganese (Mn) coordination sphere present in the newly constructed metalloproteins Mn-CH2E and Mn-CH2EY. The 2Fo–Fc maps are shown in grey and contoured at 2 σ. These structures show that a single point mutation in the secondary sphere can diversify the metal-binding coordination sphere. Oxygen atoms are shown in red; nitrogen atoms are shown in blue. [Reprinted by permission from Springer Nature from Rittle, J., et al. 2019. “An Efficient, Step-Economical Strategy for the Design of Functional Metalloproteins,” Nature Chemistry 11, 434–41. Copyright 2019.]
The Summary
Although numerous strategies have been used to create new metalloproteins, pre-existing knowledge of the tertiary and quaternary protein structure is often required to generate suitable platforms for robust metal coordination and activity. F. A. Tezcan (University of California, San Diego) and colleagues recently reported an alternative and easily-implemented approach using metal active sites by covalent tethering, in which folded protein building blocks are linked by a single disulfide bond to create diverse metal coordination environments within novel protein-protein interfaces (MASCoT). Metalloproteins generated using this method uniformly bind a wide array of first-row transition metal ions [manganese (Mn+2), iron (Fe+2), cobalt (Co+2), nickel (Ni+2), copper (Cu+2), and zinc (Zn+2)] with physiologically relevant thermodynamic affinities.
The researchers were able to create two new metalloproteins with stable Mn binding sites. Structures were obtained using X-ray crystallography data collected on Stanford Synchrotron Radiation Lightsource (SSRL) beamline 12-2 using Mn multi-wavelength anomalous diffraction (MAD) phasing (see figure). The Mn coordination sphere in Mn-CH2E includes (1) three meridional histidine side chains and (2) a single glutamate that completes a square-planar ligand arrangement around a trans-(OH2)2Mn+2 unit. These aquo ligands are in turn engaged in strong hydrogen-bonding interactions with the two other engineered histidine and glutamate side chains. By contrast, the Mn+2 coordination sphere determined for Mn–CH2EY includes all four designed histidine residues and a bound glutamate, collectively reminiscent of the nonheme Fe site found in the photosynthetic reaction center of Rhodobacter sphaeroides.
New metalloproteins were also created with other metals that could, for example, bind small gaseous molecules. The fact that all of these functional features would be difficult to design on their own but are simultaneously achieved through MASCoT with minimal engineering attests to the expediency of the covalent tethering strategy. Since this strategy is predicated on the use of well-folded protein building blocks and natural amino acids, its application in the laboratory evolution of enzymatically active metalloproteins involved in oxidative and hydrolytic processes can be realized. It may become an integral part of synthetic biology approaches.
Related Links
References
Rittle, J., M. J. Field, M. T. Green, and F. A. Tezcan. 2019. An efficient, step-economical strategy for the design of functional metalloproteins. Nature Chemistry 11(23), 434-441. [DOI:10.1038/s41557-019-0218-9]
