Automation and High-Throughput Screening Speeds Lipid Nanoparticle Development
06/06/2023
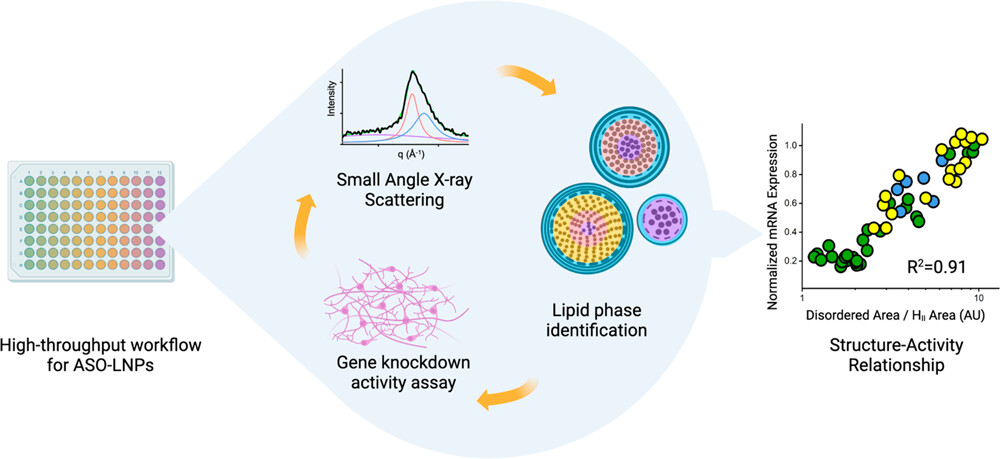
Small-Angle X-Ray Scattering Facilitates High-Throughput Screening of Lipid Nanoparticles. An improved method for developing and improving lipid nanoparticles (LNPs) includes an automated LNP formulation system (left), structural analysis by small-angle X-ray scattering (SAXS), and in vitro assessment of LNP activity (center). The results yield an association between LNP structure and activity (right) used to optimize LNP formulation.
[Reprinted under a Creative Commons license (CC BY 4.0) from Hammel et al. 2023. DOI:10.1021/acsnano.3c01186]
The Science

Diverse research areas are exploring potential applications of lipid nanoparticles (LNPs). Although pharmaceutical uses currently dominate the LNP space, as represented here by the percentage of all LNP-related scientific publications from 2000 to 2020, many other top research areas are gaining ground. BER-relevant research areas include biochemistry, biochemical methods, genetics, agriculture, and toxicology. [Reprinted under a Creative Commons license (CC BY 4.0) from Tenchov et al. 2021. DOI:10.1021/acsnano.1c04996]
LNPs are nanoscale spherical pouches composed of a lipid membrane encapsulating various cargo for delivery to cells. Most prominently, LNPs serve as delivery systems for therapeutic agents, including mRNA in two SARS-CoV-2 vaccines. The LNPs protect the mRNA or other cargo from enzymatic degradation inside the body and prevent it from adversely affecting non-target tissues and systems.
Beyond therapeutics, LNP applications have been extended to medical imaging, cosmetics, nutrition, and agriculture. They are also being studied as delivery systems for agrochemicals; as model membrane systems; and as nanoscale chemical reactors applied in nanotechnology and nanobiotechnology. The success of LNPs in medicine motivates further fundamental and applied LNP research in the fields of environmental science, such as metal detoxification and the manipulation of microbial communities, and materials science, such as the controlled synthesis of metal nanoparticles.
This study relied on high quality and high quantity SAXS data generated at the Structurally Integrated Biology for the Life Sciences resource, as well as cryogenic electron microscopy to verify the SAXS data.
The Impact
This method can be applied to rapidly characterize and optimize countless LNP formulations for different applications.
LNP morphology is recognized as a critical parameter governing LNP bioactivity, but structural analysis is typically not assessed due to limited accessibility and resource requirements. The high-throughput LNP formulation and screening workflow developed in this study enables researchers to produce and characterize LNPs at record speed. The study also includes the first-ever demonstration of how LNP structure correlates with the activity of its contents.
In addition, unlike other forms of X-ray diffraction on biological materials, SAXS samples do not require freezing or crystallization which can change LNP structure. SAXS also enables snapshots of LNPs at a specified timepoints to determine structural longevity.
The Summary
LNPs are comprised of four major components, allowing them to be customized in countless permutations. These components include ionizable lipids, helper phospholipids, cholesterol, and polyethylene glycol-lipids (PEG-lipids). Capitalizing on this flexibility, researchers used an automated process to rapidly generate a library of LNPs containing potentially therapeutic antisense oligonucleotides (ASO).
Structural characterization of the resulting ASO-LNPs was performed using high-throughput SAXS backed up by cryogenic electron microscopy (cryo-EM). Associating ASO-LNP SAXS signals to their respective cryo-EM features enabled identification of LNP structural parameters that can be analyzed directly from SAXS data.
The structural information was then combined with the results of a high-throughput in vitro efficacy assay in mouse cortical neurons to determine any relationship between ASO-LNP structure and cellular efficacy. The method yielded a structure–activity relationship for LNPs in a high-throughput setup to help identify chemical compositions that produce optimal LNP structures for maximized efficacy.
Funding
All small angle X-ray scattering (SAXS; solution X-ray scattering) data were collected at the Structurally Integrated Biology for the Life Sciences (SIBYLS) Advanced Light Source (ALS) beamline, which operates through support from the following sources: National Institute of Health grant ALS-ENABLE (P30 GM124169), National Cancer Institute grant SBDR (CA92584), Department of Energy through Basic Energy Science grant DE-AC02-05CH11231, and Biological and Environmental Research grant IDAT.
Related Links
- BER Resource: Structurally Integrated Biology for the Life Sciences
- Breaking Barriers in Drug Delivery with Better Lipid Nanoparticles
References
Hammel, Michal, et al. 2023. “Correlating the Structure and Gene Silencing Activity of Oligonucleotide-Loaded Lipid Nanoparticles Using Small-Angle X‑Ray Scattering,” ACS Nano 17 (12), 11454-11465. DOI:10.1021/acsnano.3c01186
Tenchov, Rumiana, et al. 2021. “Lipid Nanoparticles: From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement,” ACS Nano 15 (11), 16982-117015. DOI: 10.1021/acsnano.1c04996
