Breakthrough Shines Light on Disease-Fighting Protein
02/25/2019
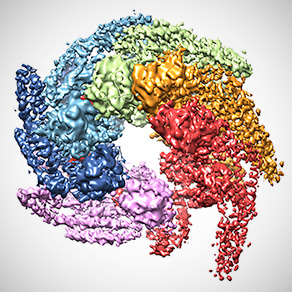
Combined crystal and cryo-em structures of Hsp104 disaggregase from the fungus Calcarisporiella thermophila. [Reprinted with permission from Elsevier from Michalska et al. 2019. “Structure of Calcarisporiella thermophila Hsp104 Disaggregase that Antagonizes Diverse Proteotoxic Misfolding Events," Structure 27(3), 449–63. Copyright 2020 Elsevier.]
The Summary
A team of researchers have obtained the highest-resolution structure of the fungal protein Hsp104, which could be used to hinder the formation of certain degenerative diseases. Using the Advanced Photon Source at Argonne National Laboratory, the researchers combined X-ray crystallography and cryo-electron microscopy (cryo-EM) to also verify that these protein-formed hexamers, once believed flat, have a helical structure. Known as a chaperone, the hexameric AAA+ protein Hsp104 helps in the natural folding processes of proteins for proper cell function and may be able to repair misfolded or aggregated proteins that can lead to protein-caused abnormalities.
Related Links
- BER Resource: Stanford-SLAC Cryo-EM Center
- BER Resource: Structural Biology Center
- Feature Story: Breakthrough shines light on disease-fighting protein
References
Michalska, K., et al. 2019. “Structure of Calcarisporiella thermophila Hsp104 Disaggregase that Antagonizes Diverse Proteotoxic Misfolding Events.” Structure 27(3), 449–63. [DOI:10.1016/j.str.2018.11.001]
