Characterization of Elusive Yeast Protein Complex Involved in Autophagy
11/07/2017
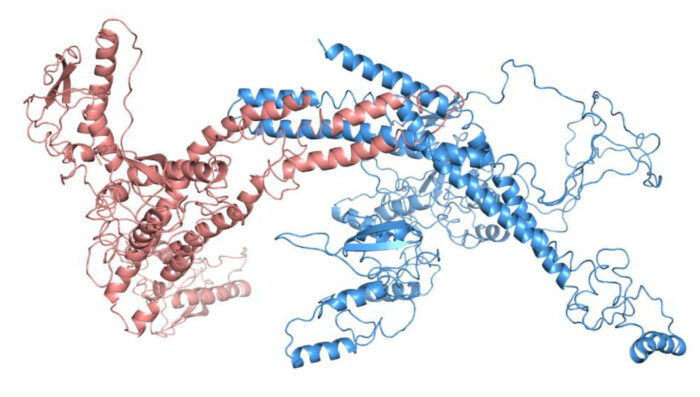
Autophagy machinery in yeast. Scientists used solution X-ray spectroscopy (SAXS) to develop a structural model of two relatively uncharacterized components of the Atg1 kinase complex, a protein complex involved in the initiation of autophagy. The two protein components, Atg20 (blue) and Snx4 (red), are necessary for successful induction of autophagy. [Reprinted with permission from Popelka, H., et al. 2017. "Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction." PNAS, 114 (47), E10112–E10121. DOI:10.1073/pnas.1708367114.]
The Summary
Autophagy is a form of cellular self-cleaning, where cytoplasmic material destined for degradation is captured in double-membrane compartments that mature and fuse with a degradative organelle. Autophagy is essential to maintain cellular homeostasis, respond to cellular stress, and prevent the accumulation of material that could damage the cell.
Highly dynamic proteins engaged in autophagy are very challenging to study using standard techniques that reveal a 3D structure. Autophagy is initiated by the Atg1 protein complex. Whereas recent work has provided functional and mechanistic insight into many components of the Atg1 complex, one member of this complex—Atg20—has remained relatively uncharacterized.
Scientists used small-angle X-ray scattering (SAXS) at the LiX beamline at NSLS-II to provide the first insight into the structure and function of Atg20 in Saccharomyces cerevisiae yeast, including identifying an amphipathic helix required for efficient autophagy and membrane tubulation.
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: Scientists uncovered an elusive structure and function of a protein complex involved in autophagy
References
H. Popelka, A. Damasio, J. E. Hinshaw, D. J. Klionsky, and M. J. Ragusa. “Structure and function of yeast Atg20, a sorting nexin that facilitates autophagy induction.” PNAS, 2017, E10112–E10121. DOI: 10.1073/pnas.1708367114
