Complexation of Metals with Bacterial Methanobactins
06/02/2021
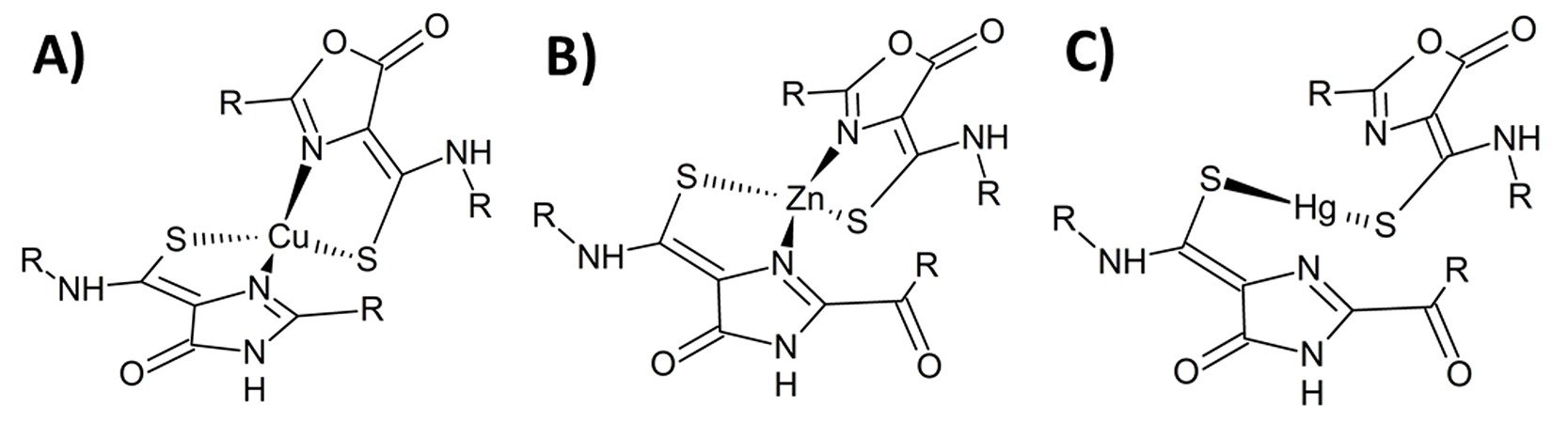
Spectroscopic techniques can reveal coordination geometry—the geometrical pattern of atoms bound to an atom of interest in a molecule. Here (figure A), the coordination geometry of copper (Cu) complexed with a methanobactin from Methylocystis sp. strain SB2 was observed in crystal structures. Researchers then proposed the structure of other transition metals in complex with the methanobactin, including zinc (Zn) (figure B) from spectroscopic data and mercury (Hg) (figure C) from spectroscopic and EXAFS data. [Reprinted with permission from Elsevier from Eckert, P., et al. 2021. “Spectroscopic and Computational Investigations of Organometallic Complexation of Group 12 Transition Metals by Methanobactins from Methylocystis sp. SB2,” Journal of Inorganic Biochemistry 223, 111496. Copyright 2021.]
The Science
Spectroscopic techniques and time-dependent density functional theory (TD-DFT) calculations shed light on the structure and geometry of bacterial methanobactins binding to the transition metals Zn, Cd, and Hg.
The Impact
The results lay the foundation for future work exploring the impact of methanobactins on the speciation and biogeochemical cycling of transition metals, including highly toxic methylmercury.
Summary
Methanobactins are small peptides secreted by methanotrophic bacteria to facilitate their acquisition of Cu, an element they need to catalyze aerobic oxidation of methane to methanol. These methanobactins can also bind other trace metals, including the group 12 transition metals Zn, Cd, and Hg. Therefore, the structure of methanobactin-metal complexes has implications for the transport, bioavailability, and toxicity of trace metals in the environment.
This study sought to understand the structure and geometry of methanobactins binding to Zn, Cd, and Hg. The complexation of these metals by methanobactin from Methylocystis sp. strain SB2 was studied using a combination of absorbance, fluorescence, extended x-ray absorption fine structure (EXAFS) spectroscopy, and TD-DFT calculations.
Collectively, the results represent the first combined computational and experimental spectroscopy study of methanobactins and shed new light on molecular interactions and dynamics that characterize complexes of methanobactins with Group 12 transition metals.
Research Details
- Methanobactin was isolated from the bacterium Methylocystis sp. strain SB2, purified, and mixed with metal salt solutions. Various ratios of methanobactin to metal, plus aging experiments for time resolved data, were studied.
- Techniques included Hg L3-edge EXAFS in combination with UV-vis and fluorescence spectroscopy and TD-DFT calculations, providing a comprehensive overview of peptide-metal interactions.
- Absorption and fluorescence spectroscopies indicated the formation of dimeric complexes that become monomers when the ratio of methanobactin to metal is equivalent.
- Download technical summary
Related Links
- BER Resource: Structural Molecular Biology Resource
- U.S. EPA. 2013. 2011 National Listing of Fish Advisories (Technical Fact Sheet). U.S. Environmental Protection Agency.
- U.S. EPA. 2011. 2010 Biennial National Listing of Fish Advisories (Technical Fact Sheet). U.S. Environmental Protection Agency
References
Eckert, P., Johs, A., Semrau, J.D., DiSpirito, A.A., Richardson, J., Sarangi, R., Herndon, E., Gu, B., Pierce, E.M. “Spectroscopic and computational investigations of organometallic complexation of group 12 transition metals by methanobactins from Methylocystis sp. SB2.” 2021. Journal of Inorganic Biochemistry. [DOI: 10.1016/j.jinorgbio.2021.111496]
