Crystal Structure of NOV1 Enzyme Reveals Mechanism of Action
11/30/2016
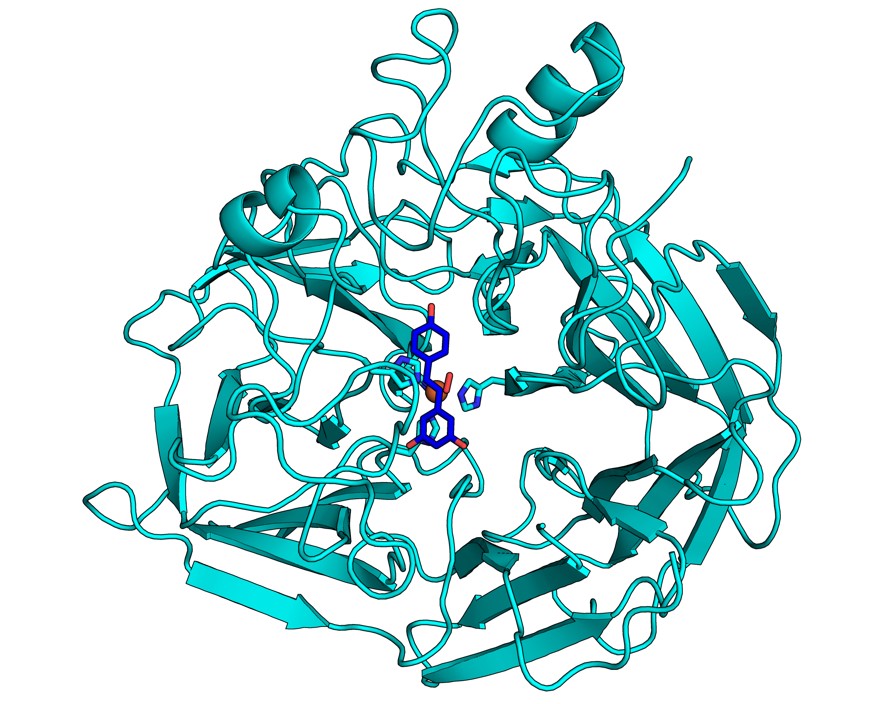
The crystal structure of NOV1, a stilbene-cleaving oxygenase, reveals features of the enzyme at atomic resolution, including the positions of iron (orange ball behind stick drawing), dioxygen (red), and a representative substrate (blue) in the active site of the enzyme. (Credit: Ryan McAndrew/JBEI and Lawrence Berkeley National Laboratory.)
Stilbenes are compounds produced naturally in a wide variety of plant species and some bacteria, and are also derived from lignin during the kraft pulping process. Enzymes capable of converting stilbenes into useful chemicals or fuels could positively impact the economics of lignocellulosic biomass processing.
Collaborators from two U.S. Department of Energy Bioenergy Research Centers report the atomic-level structure of NOV1, a stilbene-cleaving oxygenase (SCO) capable of breaking down the stilbene substrate resveratrol into two smaller compounds. Enzymes such as NOV1 could prove valuable in the biological production of important molecular fragments derived from lignin.
Funding Acknowledgements
Berkeley Center for Structural Biology (BCSB), Advanced Light Source (ALS), Lawrence Berkeley National Laboratory (LBNL), work performed as collaboration between the Joint BioEnergy Institute (JBEI; https://www.jbei.org) and Great Lakes Bioenergy Research Center (GLBRC; https://www.glbrc.org). JBEI support: Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE) Office of Science, through contract DE-AC02-05CH11231 between LBNL and DOE. GLBRC support: OBER, DOE Office of Science, through Grant DE-FG02-07ER64495. BCSB support in part: National Institutes of Health’s (NIH) National Institute of General Medical Sciences (NIGMS). ALS support: Director, Office of Basic Energy Sciences (OBES), DOE Office of Science, under Contract DE-AC02-05CH11231. Support for part of work: National Science Foundation (NSF) under Cooperative Agreement 1355438.
Related Links
- BER Resource: Structurally Integrated Biology for the Life Sciences
- Feature Story: New crystallography finding by JBEI and GLBRC benefits bioenergy industry
References
McAndrew, R.P., N. Sathitsuksanoh, M.M. Mbughuni, R.A. Heins, J.H. Pereira, A. George, K.L. Sale, B.G. Fox, B.A. Simmons, and Paul D. Adams. 2016. “Structure and mechanism of NOV1, a resveratrol-cleaving dioxygenase” PNAS 113 (50) 14324-14329. doi:10.1073/pnas.1608917113
