Crystal Structures of SARS-CoV-2 Yield Understanding of Viral Replication Engine
04/17/2020
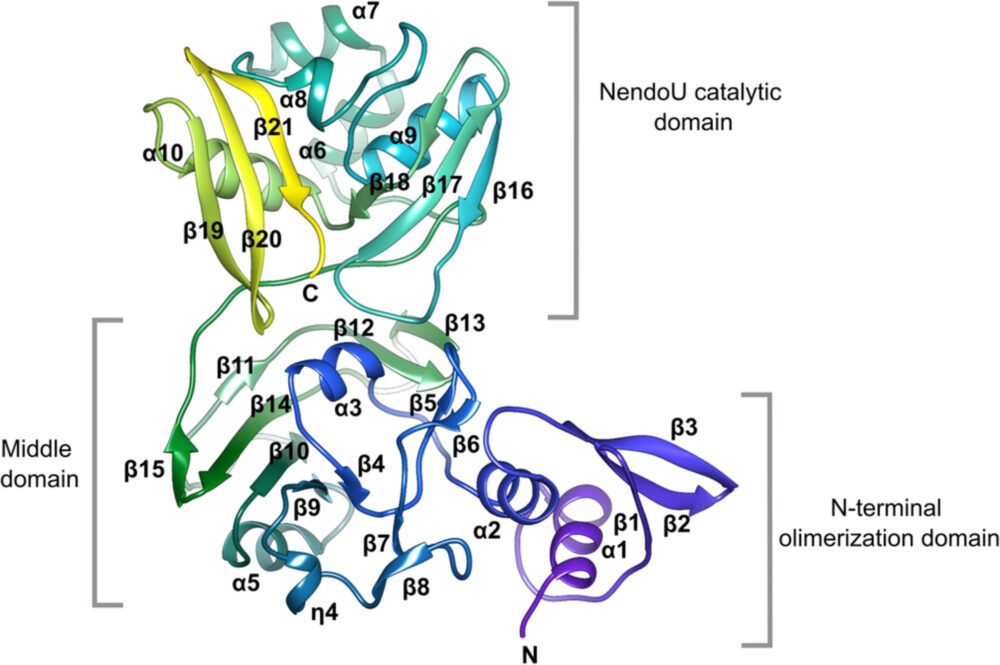
Structure of a single monomer of the Nsp15 hexamer produced by SARS-CoV-2, which plays a role in virulence. [Reprinted under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International https://creativecommons.org/licenses/by-nc-nd/4.0/ from Youngchang et al. 2020. DOI: 10.1002/pro.3873]
The rapid upsurge and proliferation of SARS-CoV-2 raised questions about how the virus became so much more transmissible than the SARS-CoV-1 and MERS-CoV coronaviruses. Although the viruses are similar, detailed information about SARS-CoV-2 protein structures and functions is needed to rapidly develop effective vaccines, antibodies, and antivirals.
Scientists applied a high-throughput protein production and structure determination pipeline to produce SARS-CoV-2 proteins and structures. They report two high-resolution crystal structures of nonstructural protein 15 (Nsp15), an endoribonuclease conserved across coronaviruses that processes viral RNA to evade detection by host defense systems.
The Nsp15 structure is very similar to the SARS-CoV-1 and MERS-CoV homologs, but shows some differences that may contribute to its greater virulence.
Related Links
- BER Resource: Structural Biology Center
- Feature Story: New Structures of SARS-CoV-2 Lead to Improved Understanding of Viral Replication Engine
References
Youngchang Kim, Robert Jedrzejczak, Natalia I. Maltseva, Mateusz Wilamowski, Michael Endres, Adam Godzik, Karolina Michalska, and Andrzej Joachimiak. “Crystal structure of Nsp15 endoribonuclease NendoU from SARS-CoV-2,” Prot. Sci. 29, 1596 (2020). [DOI: 10.1002/pro.3873]
