Description of Hydration Water in Green Fluorescent Protein Solution
10/06/2016
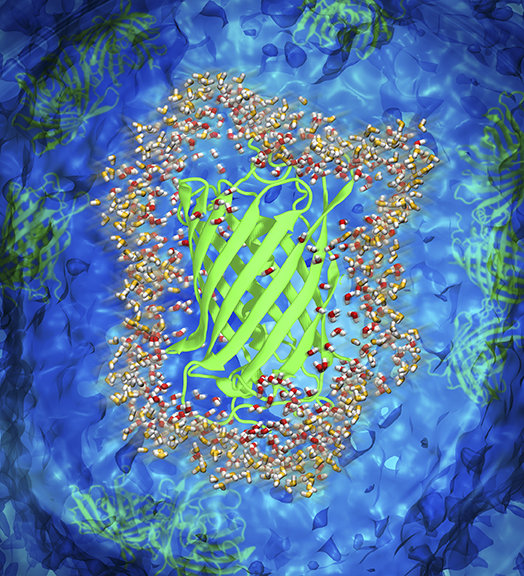
Graphic representation of hydration water surrounding green fluorescent protein. [Reprinted with permission from Perticaroli, S., et al. “Description of Hydration Water in Protein (Green Fluorescent Protein) Solution.” J. Am. Chem. Soc. 139(3), 1098–1105 (2017). [DOI:10.1021/jacs.6b08845]. Copyright 2016 American Chemical Society.]
Many unanswered questions remain about how macromolecules in solution perturb the water molecules in which they are immersed. Scientists used neutron scattering to provide an experimental description of the dynamical perturbation of water molecules surrounding proteins in solution, which play an important role in protein function and stability. Quantifying the magnitude of the perturbation of water around the green fluorescent protein (GFP), they found a systematic length-scale dependence of the dynamical retardation factor compared to the perturbation of bulk water. The findings deepen the understanding of water perturbation, which is of practical interest to researchers in food science, personal care, pharmaceutics, and protein dynamics.
Instruments and Facilities
Neutron scattering at Center for Structural Molecular Biology and Spallation Neutron Source at Oak Ridge National Laboratory.
Funding Acknowledgements
H.O’N. and Q.Z. support: Center for Structural Molecular Biology, funded by the Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE) Office of Science, under Contract FWP ERKP291. Research at Oak Ridge National Laboratory’s (ORNL) Spallation Neutron Source (SNS) sponsored by the Office of Basic Energy Sciences (OBES) Scientific User Facilities Division, DOE Office of Science. ORNL facilities sponsored by UT-Battelle, LLC, for the DOE under Contract No. DEAC0500OR22725.
Related Links
References
Perticaroli, S., et al. “Description of Hydration Water in Protein (Green Fluorescent Protein) Solution.” J. Am. Chem. Soc. 139(3), 1098–1105 (2017). [DOI:10.1021/jacs.6b08845].
