Detailing the Molecular Roots of Alzheimer’s Disease
12/20/2016
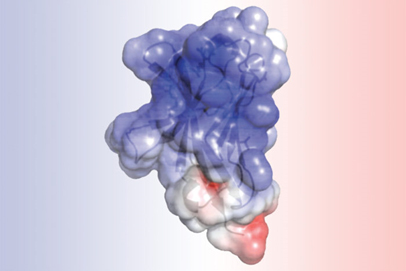
TREM2 is a protein involved in Alzheimer’s disease and other neurodegenerative disorders. Its electrostatic surface structure, depicted here, was determined at the Structural Biology Center X-ray facility at the Advanced Photon Source at Argonne National Laboratory. [From Kober, D. L., et al. “Neurodegenerative Disease Mutations in TREM2 Reveal a Functional Surface and Distinct Loss-of-Function Mechanisms.” eLife 5, e20391 (2016). DOI:10.7554/eLife.20391. Reused under a Creative Commons license (CC BY 4.0, https://creativecommons.org/licenses/by/4.0/).]
Scientists have captured the three-dimensional (3D) structure of a molecule implicated in Alzheimer’s disease. Knowing the shape of this molecule—and how that shape may be disrupted by genetic mutations—can help in understanding how Alzheimer’s and other neurodegenerative diseases develop and ways to prevent and treat them.
Certain mutations that alter the structure of the molecule TREM2 are associated with an increased risk of developing late-onset Alzheimer’s, frontal temporal dementia, Parkinson’s disease, and sporadic amyotrophic lateral sclerosis (ALS), indicating that TREM2 may be involved in cognitive decline. Other TREM2 mutations are linked to Nasu-Hakola disease, a rare inherited condition that causes progressive dementia and death in most patients by age 50. Although its exact contribution is unknown, dysfunctional TREM2 correlates with neurodegeneration, and inflammation appears to be the common thread in neurodegenerative conditions.
Structural analysis of TREM2 revealed that the mutations associated with Alzheimer’s alter the surface of the protein, while those linked to Nasu-Hakola influence the “guts” of the protein. The difference in location could explain the severity of Nasu-Hakula, in which signs of dementia begin in young adulthood. The internal mutations totally disrupt the structure of TREM2, resulting in fewer TREM2 molecules. The surface mutations, in contrast, leave TREM2 intact but likely make it harder for the molecule to connect to proteins or send signals as normal TREM2 molecules would.
TREM2 lies on the surface of immune cells called microglia, which are thought to be important “housekeeping” cells involved in, for example, maintaining healthy brain biology via a process called phagocytosis that cleans cellular waste, including the amyloid beta that is known to accumulate in Alzheimer’s disease. If the microglia lack TREM2 or the TREM2 is dysfunctional, these cellular housekeepers can’t perform their cleanup tasks.
Though the exact function of TREM2 is still unknown, mice without TREM2 have defects in their microglia. With these structural data, scientists can study how TREM2 works, or doesn’t work, in these neurodegenerative diseases, as well as other inflammatory conditions including chronic obstructive pulmonary disease and stroke. The structure of TREM2 could be important for understanding many chronic and degenerative diseases.
Instruments and Facilities
X-ray facility at the Advanced Photon Source (APS) at Argonne National Laboratory.
Funding Acknowledgements
Daniel L Kober: American Heart Association (Predoctoral Fellowship PRE22110004) and National Institute of General Medical Sciences (T32-GM007067); Jennifer M Alexander-Brett: National Heart, Lung, and Blood Institute (K08-HL121168) and Burroughs Wellcome Fund (Career Award for Medical Scientists); Celeste M Karch: National Institute on Aging (K01-AG046374); Carlos Cruchaga: National Institute on Aging (R01-AG044546); Marco Colonna: National Institute on Aging (R01-AG051485); Michael J Holtzman: National Heart, Lung, and Blood Institute (R01-HL120153) and National Heart, Lung, and Blood Institute (R01-HL121791); and Thomas J Brett: National Heart, Lung, and Blood Institute (R01-HL119813), National Institute on Aging (P50-AG005681-30.1), Knight Alzheimer’s Disease Research Center (Pilot grant P50-AG005681-30.1), Alzheimer’s Association (AARG-16-441560). Acknowledgements: Support in part: TJB: National Institutes of Health (NIH; R01-HL119813, Knight Alzheimer’s Disease Research Center pilot grant P50-AG005681-30.1, Alzheimer’s Association Research Grant AARG-16-441560); JAB: K08-HL121168 and Burroughs-Wellcome Fund Career Award for Medical Scientists; CMK: NIH K01-AG046374; CC: R01-AG044546; MC: R01-AG051485; MJH: R01-HL120153 and R01-HL121791; DLK: T32-GM007067 and American Heart Association Predoctoral Fellowship PRE22110004. Results derived from work performed at Argonne National Laboratory (ANL) Structural Biology Center (SBC). ANL is operated by University of Chicago Argonne, LLC, for the Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE) Office of Science (DE-AC02-06CH11357).
Related Links
- BER Resource: Structural Biology Center
- Feature Story: Study details molecular roots of Alzheimer’s
- Press Release by the Advanced Photon Source
References
Kober, D. L., et al. “Neurodegenerative Disease Mutations in TREM2 Reveal a Functional Surface and Distinct Loss-of-Function Mechanisms,” eLife 5, e20391 (2016). [DOI:10.7554/eLife.20391].
