Essential Enzyme in Bacterial Protein Synthesis
05/21/2021
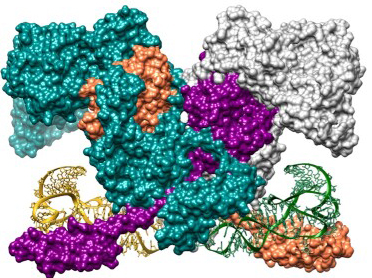
X-ray macromolecular crystallography reveals the structure and mode of interaction of the enzyme phenylalanyl-tRNA synthetase, which is integral to protein synthesis in the pathogenic bacterium Mycobacterium tuberculosis. The enzyme (its four subunits shown in aqua, orange, purple, and white) catalyzes the attachment of the amino acid phenylalanine to its corresponding transfer RNA (yellow and green ribbon models), playing an essential role in translating the DNA code into protein sequence. [Reprinted under a Creative Commons Attribution-Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) from Michalska K, et al. "Mycobacterium tuberculosis Phe-tRNA synthetase: structural insights into tRNA recognition and aminoacylation," Nucleic Acids Res. 49(9):5351-5368. DOI: 10.1093/nar/gkab272.]
In recent work using the Structural Biology Center X-ray beamline 19-ID at the U.S. DOE’s Advanced Photon Source, researchers gained important insights into the structure and mechanism of phenylalanyl-tRNA synthetase, a critical enzyme involved in protein synthesis in Mycobacterium tuberculosis.
Multi-drug resistant and extensively drug-resistant strains of M. tuberculosis have become a major global problem, increasing the need for new antibiotics. This has driven researchers to seek new bacterial targets for structure-based drug design.
The work, published in Nucleic Acids Research, will form the basis of structure-based drug design efforts aimed at exploiting the unique features of the enzyme, a method that has been successful in developing pharmaceuticals against methicillin-resistant Staphylococcus aureus (MRSA) and some fungal infections.
Related Links
- BER Resource: Structural Biology Center
- Feature Story: Using Protein Structural Information to Understand the Mechanism of an Essential Enzyme for Fighting Tuberculosis
References
Michalska K, Jedrzejczak R, Wower J, Chang C, Baragaña B, Gilbert IH, Forte B, Joachimiak A. “Mycobacterium tuberculosis Phe-tRNA synthetase: structural insights into tRNA recognition and aminoacylation,” Nucleic Acids Res. 49(9):5351-5368. [DOI: 10.1093/nar/gkab272]
