Fine Tuning Drugs to Fight Cancer
11/30/2020
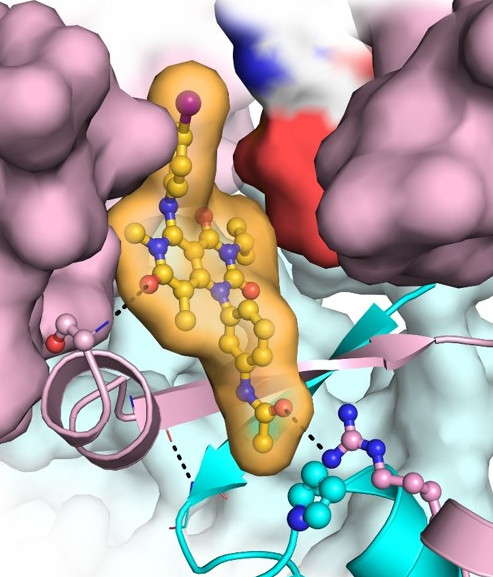
Schematic of the drug trametinib (orange), used to treat melanoma, as it binds to its target methyl ethyl ketone (pink) and and its scaffold protein, kinase suppressor of Ras (cyan). [Image credit: Z. M. Khan, et al., Icahn School of Medicine at Mount Sinai.]
The Science
Scientists reveal how the drug trametinib binds itself to its target. Co-crystal structures of the clinical methyl ethyl ketone (MEK) inhibitor, trametinib, demonstrate that the drug binds at the interface of MEK and its scaffold protein, kinase suppressor of Ras (KSR).
The Impact
Although trametinib is used to treat melanoma, its mechanism of action was not fully understood; this work reveals how the drug binds to its target.
Summary
A broad range of cancers, including pancreatic, skin, and colorectal cancer, are driven by abnormal activity in the mitogen activation protein kinase network and are linked to frequent mutations in K-RAS and B-RAF. Trametinib is a promising cancer drug that binds to MEK and is currently FDA-approved for the treatment of BRAF V600E/K mutant melanoma; however, how trametinib works and why this drug shows differential activity to other MEK inhibitors has been a mystery.
In this work, scientists from the Arvin Dar Laboratory at the Tisch Cancer Institute in the Icahn School of Medicine at Mount Sinai, New York, determined the first ever co-crystal structures of trametinib, revealing an unexpected mode of action whereby the drug spans the interface of an important regulatory complex in the RAS pathway.
Using crystallographic, biochemistry, and cell biology analysis, the team found that trametinib simultaneously binds to MEK and its cellular partner KSR. Further, based on these insights, the Dar laboratory designed a novel compound, which they dubbed “Trametiglue,” to overcome a common drug resistance mechanism that limits the efficacy of MEK inhibitors as cancer therapeutics.
This work reveals a novel strategy for designing compounds that bind at the interface of protein-protein complexes, which holds great potential for many challenging drug targets.
For this study the X-ray diffraction data were collected at the Highly Automated Macromolecular Crystallography (AMX) and Frontier Microfocusing Macromolecular Crystallography (FMX) beamlines at the National Synchrotron Light Source II (NSLS-II). NSLS-II is a U.S. Department of Energy (DOE) Office of Science User Facility located at DOE’s Brookhaven National Laboratory and offers access to state-of-the-art materials characterization and life science tools for researchers from industry and academia. Crystallography data were also collected at the LS-CAT beamline at the Advanced Photon Source at Argonne National Laboratory.
Related Links
- BER Resource: Center for BioMolecular Structure
- BER Resource: Structural Biology Center
- Feature Story: Fine Tuning Drugs to Fight Cancer
References
Z. M. Khan, A. M. Real, W. M. Marsiglia, A. Chow, M. E. Duffy, J. R. Yerabolu, A. P. Scopton, A. C. Dar. “Structural basis for the action of the drug trametinib at KSR-bound MEK.” Nature 588, 509–514(2020). [DOI: 10.1038/s41586-020-2760-4]
