Fusion Enzymes Improve Yield of Functionalized Terpenes
01/19/2021
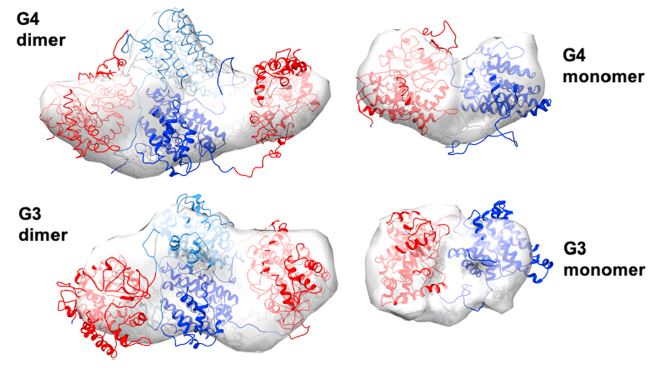
Size exclusion chromatography coupled to small angle X-ray scattering (SEC-SAXS) was used to characterize fusion enzymes comprising cytochrome P450 (red) and terpene synthase (blue). Several engineered fusion proteins, including G3 and G4 shown here in dimer and monomer forms, improved the efficiency of functionalized terpene production over unfused enzymes. [Reprinted from Metabolic Engineering Vol. 03(64), Wang et al., “Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450.” Pages 41-51, Copyright (2021), with permission from Elsevier. https://www.sciencedirect.com/journal/metabolic-engineering]
Terpenes are a large class of natural products that can be used as precursors of fuel additives, fragrances, insecticides, pharmaceuticals, and other bio-based compounds.
Enzymes such as cytochrome P450 can play an important role in the modification of terpenes essential for new bioactivities. However, the hydrophobicity and volatility of terpene molecules can limit the availability of the substrate around the enzyme and result in low enzymatic conversion during microbial production.
In this study, researchers developed a strategy to improve the accessibility of terpene molecules for the P450 reaction by linking terpene synthase and P450 together into a synthetic fusion protein that draws P450 into closer contact with its terpene substrate. Several of the engineered fusion proteins they tested significantly increased the production of oxidized terpenes.
Structural analysis of the fusion proteins was carried out using Size Exclusion Chromatography coupled to Small Angle X-ray Scattering (SEC-SAXS) at the SIBYLS beamline 12.3.1 at the Advanced Light Source. Analysis of positive and negative examples of the fusion strategy revealed key factors enabling structure-based prediction and evaluation of effective fusion enzymes.
The results suggest that developing fusion enzymes of terpene synthase and P450 presents an efficient and widely applicable strategy for improving the biosynthetic titer of functionalized products from hydrophobic terpene intermediates.
Related Links
References
Wang X, Pereira JH, Tsutakawa S, Fang X, Adams PD, Mukhopadhyay A, Lee TS. 2021. “Efficient production of oxidized terpenoids via engineering fusion proteins of terpene synthase and cytochrome P450.” Metabolic Engineering 03;64:41-51. [DOI: 10.1016/j.ymben.2021.01.004]
