How Your Body Transports Zinc to Protect Your Health
08/15/2016
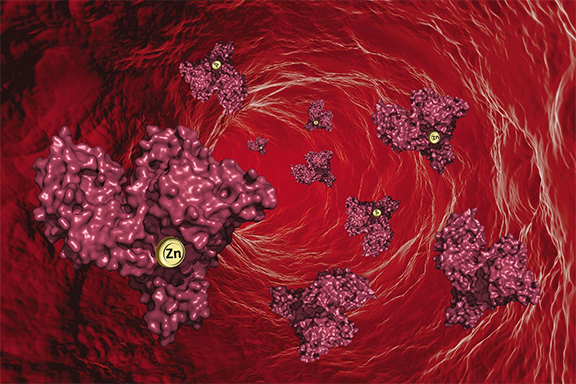
Illustration of the way zinc binds with blood serum albumin to be transported around the human body. [From Handing, K. B., et al. “Circulatory Zinc Transport Is Controlled by Distinct Interdomain Sites on Mammalian Albumins.” Chem. Sci. 7, 6635 (2016). DOI:10.1039/c6sc02267g. Published by The Royal Society of Chemistry and reused under a Creative Commons license (CC by 3.0, https://creativecommons.org/licenses/by/3.0/.)]
The Summary
Zinc is essential for wound healing, vision, DNA creation, the senses of taste and smell, and even for sexual health. But despite its importance, scientists have never fully understood the mechanism that moves the mineral through the body—until now. An international collaboration of researchers have, for the first time, created detailed blueprints of the molecular “moving vans” that ferry this important mineral through the blood everywhere it is needed. The finding gives scientists new insights into this important process and a deeper understanding of the critical role it plays in maintaining good health.
Zinc is carried through the body by a protein known as serum albumin. Scientists proved the location of the primary site where serum albumin binds with zinc, but the team also found several more secondary binding sites, revealing a more complex interaction than anticipated.
While computer models previously had been used to predict how serum albumin picks up zinc, the team used X-ray macromolecular crystallography to obtain data-based images of protein crystals showing how zinc actually bound to serum albumin. The technique allowed them to pinpoint the location of each particular zinc atom, though it was a challenging task. The schematics produced allow scientists to see, for the first time, exactly how serum albumin and zinc come together. Serum albumin also transports many other things, such as hormones and fatty acids.
Too much zinc is toxic to the body, so it must make zinc available where it is needed, but prevent harmful, excessive buildup. The study’s findings could help shed light on why certain drugs affect some patients differently than others.
Instruments and Facilities
X-ray macromolecular crystallography; Life Sciences Collaborative Access Team (LS-CAT) 21-ID-F and 21-ID-G X-ray beamlines and Structural Biology Center (SBC)-CAT 19-BM-D X-ray beamline, all at the Advanced Photon Source at Argonne National Laboratory.
Funding
Structural data used resources of the Advanced Photon Source (APS), a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory (ANL) under Contract No. DE-AC02-06CH11357. Use of LS-CAT Sector 21 supported by Michigan Economic Development Corporation and Michigan Technology Tri-Corridor (Grant 085P1000817). Work supported by NIH grants 1R01GM117325-01, 5U54GM094662-05, and R01GM053163; BBSRC grant BB/J006467/1; and British Heart Foundation grant PG/15/9/31270.
Related Links
- BER Resource: Structural Biology Center
- Feature Story: Here's how your body transports zinc to protect your health
References
Handing, K. B., et al. “Circulatory Zinc Transport is Controlled by Distinct Interdomain Sites on Mammalian Albumins.” Chem. Sci. 7, 6635 (2016). [DOI:10.1039/c6sc02267g].
