Major Scaffold Component in Nuclear Pore Complex
04/02/2013
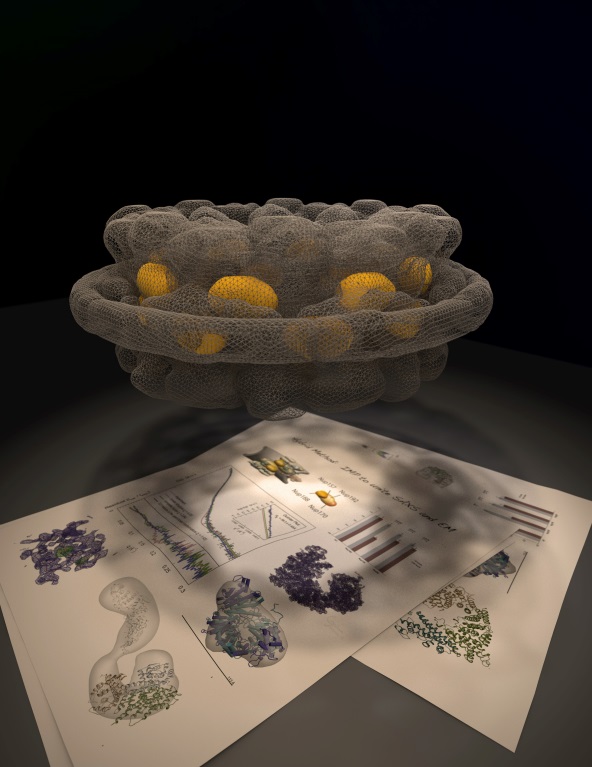
The Nuclear Pore Complex (NPC) is a 50 MDa macromolecular assembly solely responsible for the transport of macromolecules across the nuclear membrane within the eukaryotic cell. The core structure of the NPC (shown as a wire-mesh) is made up of two outer rings, two inner rings and a membrane ring. A hybrid method approach was used to determine the detailed architecture of the NPC combining structural data from crystallography, small-angle x-ray scattering, normal mode analysis, molecular dynamics simulation and electron microscopy as well as information from functional and biochemical studies.
Nuclear pore complexes (NPC) are large, octagonally symmetric, cylindrical macromolecular assemblies formed at the fusion of the inner and outer nuclear envelope membranes in eukaryotes. The NPC is responsible for the rapid, active, and selective exchange of macromolecules between the nucleus and cytoplasm. As both the gatekeepers of nucleocytoplasmic transport and a platform for the organization of numerous nuclear activities, NPCs contribute to the regulation of myriad physiological processes, including gene expression, cell cycle control, and spindle and kinetochore assembly.
NPCs are composed of proteins termed nucleoporins (Nups), forming an annular structure composed of the nuclear ring, cytoplasmic ring, a membrane ring, and two inner rings. Nup192 is a major component of the NPC’s inner ring. Small angle X-ray scattering (solution X-ray scattering) and electron microscopy (EM) studies determined the crystal structure of Saccharomyces cerevisiae Nup192 residues 2–960 (ScNup192(2–960)), and revealed that ScNup192(2–960) can undergo long-range transition between “open” and “closed” conformations.
Researchers then obtained a structural model of full-length ScNup192 based on EM, the structure of ScNup192(2–960), and homology modeling. Evolutionary analyses using the ScNup192(2–960) structure suggest that NPCs and vesicle-coating complexes are descended from a common membrane-coating ancestral complex. Finally, experimental suppression of Nup192 expression led to compromised nuclear transport, suggesting a possible role for Nup192 in modulating the permeability of the NPC central channel and ensuring efficient nuclear transport.
Funding Acknowledgements
Funding for NYSGXRC and NYSGRC: National Institutes of Health (NIH) Grants U54 GM074945 (S.K.B.) and U54 GM094662 (S.C.A.), respectively. Additional funding: NIH grants R01 GM062427 (M.P.R.), R01 GM083960 (A.S.), and U54 GM103511 and U01 GM098256 (A.S. and M.P.R.). Publication in part by Center for Synchrotron Biosciences (CSB; National Synchrotron Light Source II [NSLS-II], Brookhaven National Laboratory [BNL]) grant, P30-EB-009998, from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). Use of BNL supported by the U.S. Department of Energy (DOE) under Contract No. DE-AC02-98CH10886. Use of APS, ANL, supported by DOE. Access to LRL-CAT beam line at APS provided by Eli Lilly, which operates the facility. Portions of this research carried out at SSRL, SLAC National Accelerator Laboratory (SLAC), operated for DOE by Stanford University. The SSRL Structural Molecular Biology Program (SMBP) is supported by the DOE Office of Biological and Environmental Research (OBER), the NIH National Center for Research Resources (NCRR) Biomedical Technology Program (P41RR001209), and the NIH National Institute for General Medical Sciences (NIGMS; P41GM103393). Investigation conducted in facility constructed with support from NIH NCRR Research Facilities Improvement Program Grant number C06 RR017528-01-CEM.
Related Links
References
Sampathkumar, S. J. Kim, P. Upla, W. J. Rice, J. Phillips, B. L. Timney, U. Pieper, J. B. Bonanno, J. Fernandez-Martinez, Z. Hakhverdyan, N. E. Ketaren, T. Matsui, T. M. Weiss, D. L. Stokes, J. M. Sauder, S. K. Burley, A. Sali, M. P. Rout and S. C. Almo, “Structure, Dynamics, Evolution, and Function of a Major Scaffold Component in the Nuclear Pore Complex”, Structure 21, 560 (2013). [doi: 10.1016/j.str.2013.02.005]
