Mapping the Migration of Genetic Material
11/15/2016
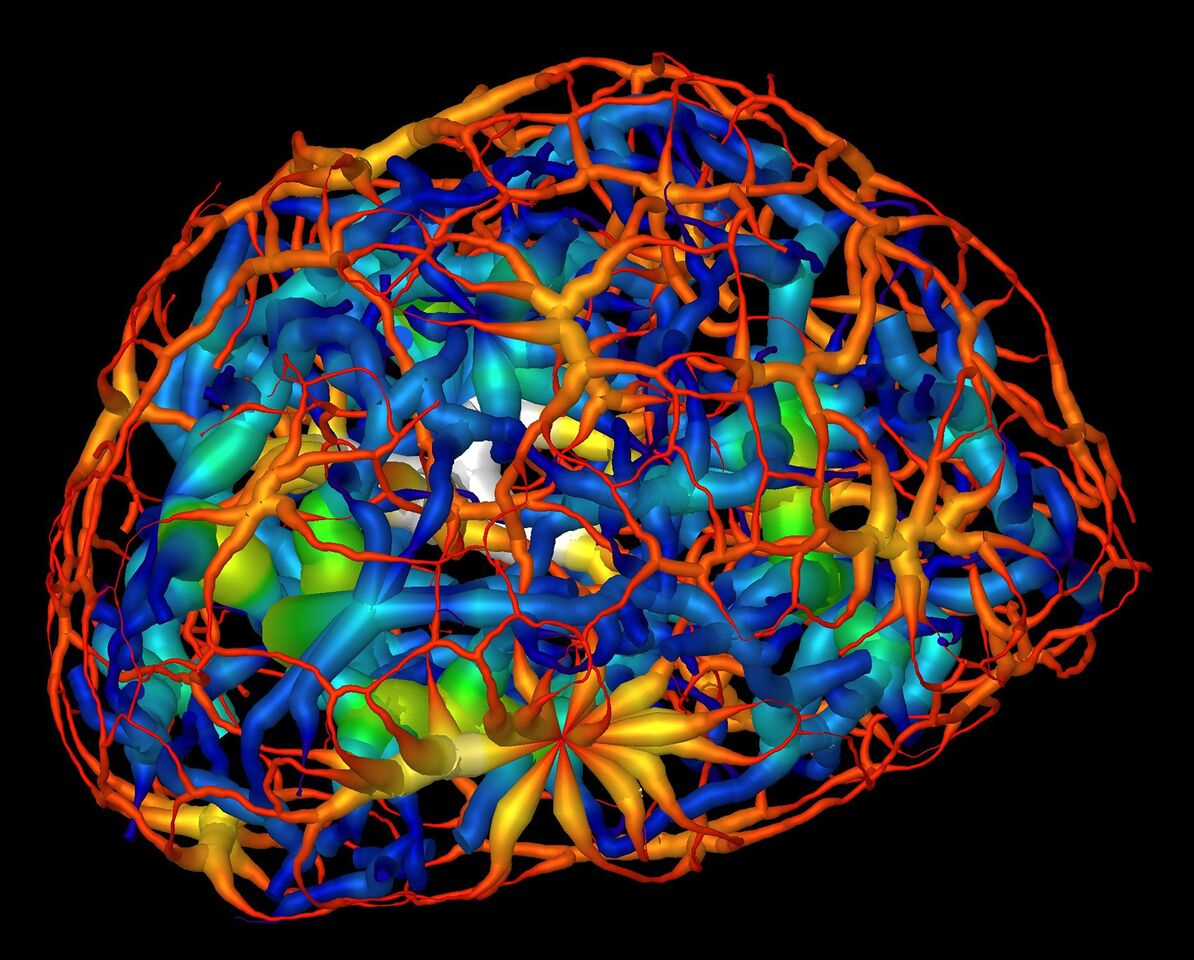
This computer rendering shows the skeletonized structure of heterochromatin (red represents a thin region ; white represents a thick region), a tightly packed form of DNA surrounding another form of DNA-carrying material known as euchromatin (dark blue represents a thin region; yellow represent the thickest) in a mouse’s mature nerve cell. [Courtesy Lawrence Berkeley National Laboratory and University of California, San Francisco.]
The Summary
Researchers used a powerful soft X-ray microscope to capture tomographic images of the genetic material in the nuclei of nerve cells at different stages of maturity. This first quantitative analysis of nuclear organization in intact mammalian cells has generated detailed three-dimensional (3D) visualizations showing an unexpected connectivity in the genetic material called chromatin. The results could aid in understanding how the patterning and reorganization of chromatin relate to the specialization of a stem cell’s function as specific genes are activated or silenced.
Understanding the logic of nuclear architecture and how it contributes to stem cell differentiation remains challenging. Until now, imaging the nucleus was possible only indirectly by staining it and hopefully achieving an even distribution. In this work, researchers used soft X-ray microtomography to record a series of images from mouse olfactory nerve cells in three separate stages of development. This unique technique provides a new way of looking at the nucleus without chemically treating the cell, allowing visualization of intact cells in a near-native state at a resolution of about 50 nanometers.
The researchers imaged frozen cells at different stages of development from dozens of different angles, then used the images to calculate a 3D reconstruction detailing changing chromatin formations inside the nucleus. The images were collected using soft X-rays within the “water window” (i.e., 284 to 543 electronvolts). The absorption of X-rays by biomolecules is linear with biochemical composition and concentration, generating a unique X-ray linear absorption coefficient measurement for each voxel (i.e., 3D pixel analog). Thus, the researchers were able to visually distinguish between different types of chromatin. Heterochromatin, for example, appears darker than euchromatin in computer-generated tomographic orthoslices (virtual sections) through the nucleus due to its greater biomolecular concentration.
The results showed that chromatin compaction increases as the cell matures, and that condensed chromatin moves to the nuclear core during differentiation. Chromatin was thought to exist as a series of disconnected islands, but imaging showed that it compartmentalizes into two distinct regions of “crowding” that form a continuous network throughout the nucleus. Based on a comparison of these results with those of similar cells in which the gene for a heterochromatin binding protein had been inactivated (“knocked out”), the researchers concluded that the protein regulates the reorganization of chromatin in mature neurons.
Soft x-ray tomography provides a powerful method to study chromatin and nuclear architecture in vivo. There is no need for chemical fixation or sectioning, preventing a plethora of artifacts introduced by fixatives or by visualization of only thin sections. With the proven success of the imaging technique, the researchers believe it is possible to perform statistical analyses based on large collections of cell nuclei images sorted by different stages of development. Coupled with other types of imaging techniques, researchers hope to isolate individual gene-selection processes in upcoming work.
Instruments and Facilities
Soft X-ray tomography (beamline 2.1) of the Advanced Light Source at the National Center for X-Ray Tomography at Lawrence Berkeley National Laboratory.
Funding
Research reported in this publication was supported by grants from NIH (R01DA030320 and U01DA040582 to S.L. and C.A.L.). The National Center for X-ray Tomography is supported by NIH (P41GM103445) and DOE’s Office of Biological and Environmental Research (DE-AC02-5CH11231).
Related Links
- BER Resource: National Center for X-Ray Tomography
- Feature Story: Mapping the Migration of Genetic Material
References
Le Gros, M. A., et al. “Soft X-Ray Tomography Reveals Gradual Chromatin Compaction and Reorganization During Neurogenesis In Vivo,” Cell Reports 17(8), 2125–2136 (2016). [DOI:10.1016/j.celrep.2016.10.060].
