Mechanism of Activation of a Plant Metacaspase
05/07/2020
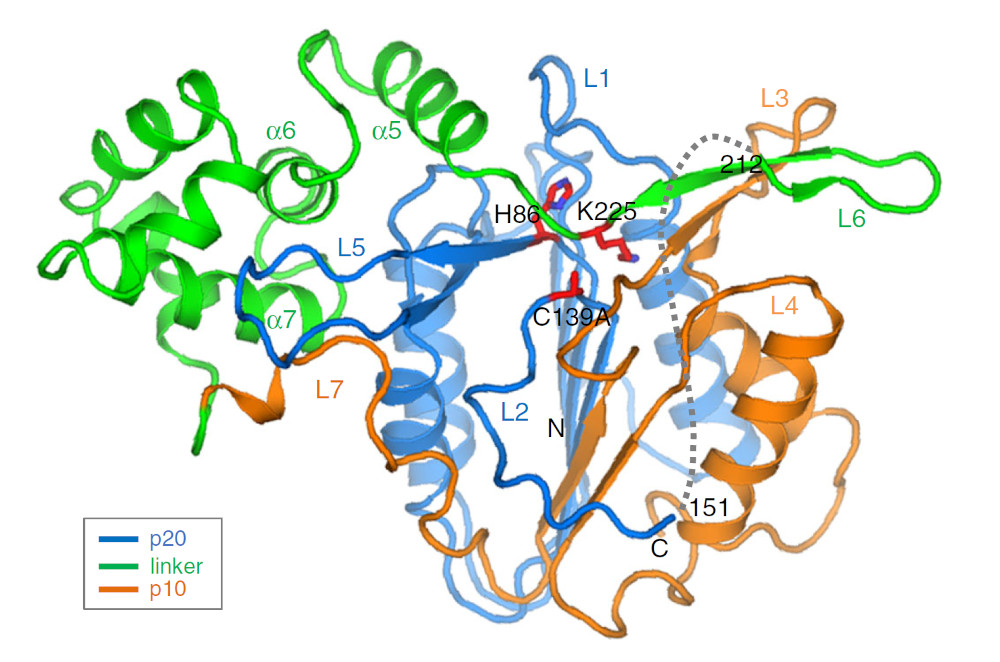
Scientists reveal a critical activation mechanism for a plant metacaspase that offers a pathway for more sustainable crops and biofuels. The AtMC4 metacaspase enzyme comprises three domains, including a “linker” domain (green). A side chain in the linker domain blocks the enzyme’s active site until activated by Ca2+, thus ensuring the tight regulation of this protease. [Reprinted under a Creative Commons Attribution 4.0 International (CC BY 4.0) license from Zhu, P., et al. 2020."Structural basis for Ca2+-dependent activation of a plant metacaspase." Nature Communications 11 (1), 2249. DOI:10.1038/s41467-020-15830-8.]
Plant metacaspase enzymes mediate many important cellular functions, including programmed cell death, stress and immune responses, and resistance to pathogens. Using X-ray macromolecular crystallography, researchers determined the crystal structures comprising Metacaspase 4 from Arabidopsis thaliana (AtMC4) and characterized its dependence on Ca2+ for activation. Understanding the mechanisms of matacaspase-mediated responses offers a basis for future bioengineering to enable the design of more sustainable crops and biofuels.
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: Revealing the Basis for Metacaspase Activation
References
P. Zhu, X.-H. Yu, C. Wang, Q. Zhang, W. Liu, S. McSweeney, J. Shanklin, E. Lam, Q. Liu, Structural basis for Ca2+-dependent activation of a plant metacaspase. Nature Communications 11 (1), 2249 (2020). [DOI: 10.1038/s41467-020-15830-8.]
