Mechanisms of Bacterial Iron Metabolism
04/01/2022
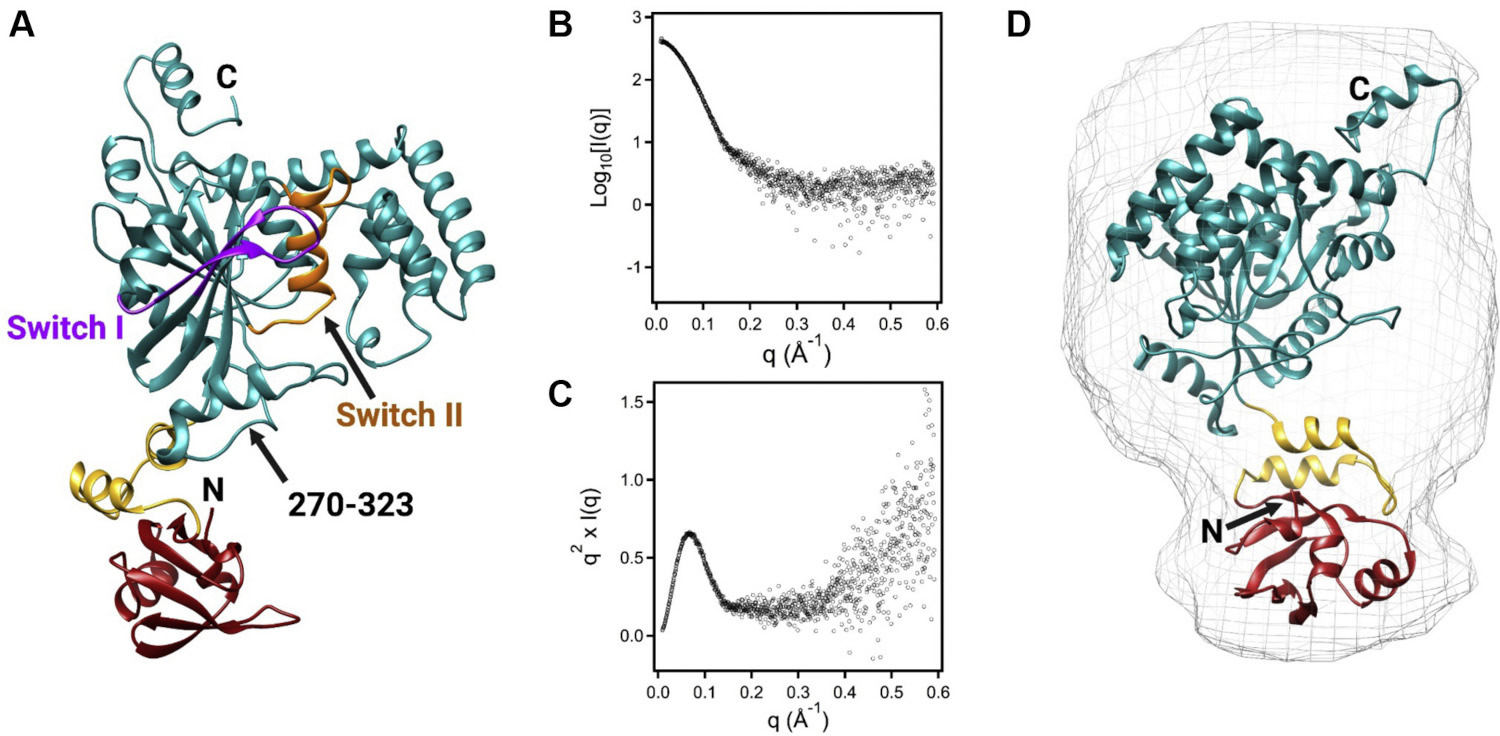
Small-angle X-ray scattering validated structural prediction of a FeoAB complex, the two constituent proteins of the prokaryotic ferrous iron transport system. (A) The predicted model of a FeoAB covalently-linked protein assembly. (B) SAXS profiles analyzed as a Kratky plot (C) show a degree of flexibility that helps explain challenges in crystallographic and cryo-EM analysis. (D) The FeoAB shape defined by SAXS (gray) overlaid on the structure prediction (colored ribbons). [Reprinted under a Creative Commons CC BY license from Sestok et al. 2022. DOI: 10.1016/j.jbc.2022.101808]
Iron (Fe) is an essential element for nearly all organisms, and under anoxic and/or reducing conditions, Fe2+ is the dominant form of iron available to bacteria. The ferrous iron transport (Feo) system is the primary Fe2+ import machinery in prokaryotic organisms, and two constituent proteins (FeoA and FeoB) are conserved across most bacterial species. However, how FeoA and FeoB function relative to one another remains enigmatic.
Using a combination of structural modeling, SAXS, and hydrogen-deuterium exchange mass spectrometry, researchers showed that FeoA and FeoB interact in a nucleotide-dependent manner and mapped the protein-protein interaction interface. The results provide insight into mechanisms of bacterial iron import and utilization.
The study also helps define a macromolecular switch that could be tuned to control iron utilization by microbes. As iron is a key constituent of many redox-sensitive reactions within microbes, the mechanism is fundamental to many systems within DOE mission space.
Funding Acknowledgements
High-throughput and SEC-SAXS data were collected on the SIBYLS beamline 12.3.1 at the Advanced Light Source (ALS), a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the Department of Energy, Office of Basic Energy Sciences, through the Integrated Diffraction Analysis Technologies (IDAT) program, supported by the DOE Office of Biological and Environmental Research. Diffraction data were collected at the Advanced Photon Source, Argonne National laboratory on LS-CAT beamline 21-ID-G. A full listing of funding sources can be found in Sestok et al. 2022.
Related Links
References
Sestok et al. 2022. A fusion of the Bacteroides fragilis ferrous iron import proteins reveals a role for FeoA in stabilizing GTP-bound FeoB. The Journal of Biological Chemistry, 298(4): 101808. [DOI: 10.1016/j.jbc.2022.101808]
