Mechanistic Insights into Cu(I) Biogenesis of Galactose Oxidase
11/02/2020
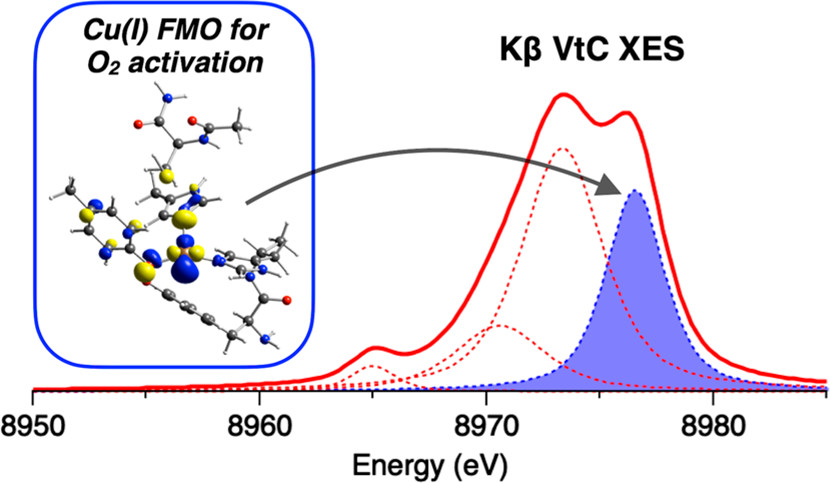
Kβ X-ray emission spectroscopy (XES) is a promising technique for investigating Cu(I) active sites in metalloproteins involved in O2 activation. By detecting photons emitted by electronic transitions from occupied orbitals, Kβ XES identified the frontier molecular orbital (FMO) in Cu(I) for O2 activation. [Reprinted with permission from Lim et al. 2020. Inorganic Chemistry, DOI:10.1021/acs.inorgchem.0c02495. Copyright 2020 American Chemical Society.]
The Science
Copper (Cu) active sites in metalloproteins, such as the secretory fungal enzyme galactose oxidase (GO), play essential roles in a wide range of biological processes. One of the most important is O2 activation, which mostly involves Cu(I) active sites. While a variety of spectroscopic methods have been utilized to provide insight into Cu(II) sites, Cu(I) sites are often considered to be “spectroscopically silent” due to their d10 closed-shell nature.
A study by Solomon et al. defines the Cu(I) frontier molecular orbital (FMO) that enables this O2 activation and demonstrates the considerable potential of Kβ X-ray emission spectroscopy (XES) in probing the FMO and key bonding interactions in spectroscopically silent Cu(I) active sites.
GO catalyzes the oxidation of primary alcohols to aldehydes. Some evidence suggests that GO may also subsequently oxidize the aldehydes to carboxylic acids. This and other research has led to the proposal that the physiological role of GO is to generate hydrogen peroxide (H2O2), perhaps as a defense against pathogenic organisms. GO is also an important component in electrochemical biosensors of galactose that are used for various biotechnology applications. It is therefore critical to understand the mechanism of Cu(I) biogenesis.
The Impact
Together with characterization of the model data, Kβ XES provides a unique opportunity to probe the interaction between Cu(I) and O for O2 activation that is not accessible with other spectroscopic techniques. In this regard, Kβ XES is expected to give important insight into the reactivity of all dioxygen activating Cu(I) metalloprotein systems (i.e., not limited to GO) and to relate the geometric structural assignment (obtained from crystallography or EXAFS methods) to unique electronic properties required for small molecule activation in metalloproteins.
Summary
Solomon et al. used Kβ XES on SSRL’s beamline 6-2 to characterize a series of Cu(I) compounds with varying biologically relevant ligand systems and compared the results to those of Cu(I) in GO to elucidate the detailed bonding interactions occurring between the metal site and the protein-based ligands. Density functional theory (DFT) calculations were also performed to evaluate the sensitivity of the spectral features to the ligand environment.
The study reveals that the Cu(I) site in preprocessed GO (GOpre) is a three-coordinate environment with a 1Tyr/2His structural model. This is consistent with previously reported EXAFS measurements by the same research group.
Related Links
References
Lim, H., et al. 2020. “Kβ X‐ray Emission Spectroscopy as a Probe of Cu(I) Sites: Application to the Cu(I) Site in Preprocessed Galactose Oxidase,” Inorganic Chemistry 59(22), 16567–581. [DOI:10.1021/acs.inorgchem.0c02495]
