Molecular Alcove Required for Anaerobic Carbon Fixation
08/01/2024
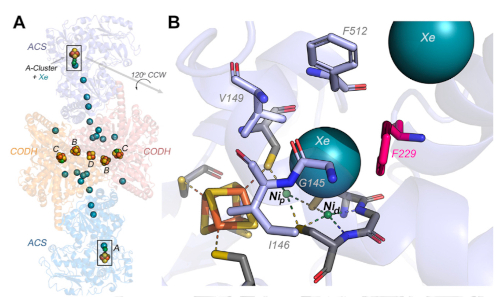
Carbon Monoxide–Binding “Alcove” Found in the Wood-Ljungdahl Pathway (WLP) of Anaerobic Carbon Dioxide Fixation. The crystal structures of the two main proteins in the WLP, carbon monoxide dehydrogenase (CODH) and acetyl-CoA synthase (ACS), help reveal several residues (panel B) that form the alcove, which helps bind CO and is crucial for enzymatic function.
[Reprinted under a Creative Commons license (CC BY 4.0) from Wiley et al. 2024. DOI:10.1016/j.jbc.2024.107503]
The Science
The anaerobic Wood-Ljungdahl pathway is one of seven known carbon fixation pathways, and the only one which conserves energy through net generation of adenosine triphosphate (ATP). At the heart of the pathway are two proteins—carbon monoxide dehydrogenase (CODH) and acetyl-CoA synthase (ACS)—which turn carbon dioxide (CO2) into acetate through a series of organometallic intermediates.
In the pathway, ACS accepts CO, produced from CO2 by CODH, and a methyl group from a cobalamin-dependent methyl transferase to form acetyl-CoA at its active site, which is composed of an iron-sulfur cluster adjacent to two nickel (Ni) atoms. CO, a critical intermediate of the pathway, travels to the active site of ACS from CODH through a series of internal gas channels, allowing the organism to tightly control this otherwise toxic substance.
In this study, researchers use X-ray absorption spectroscopy and other techniques to demonstrate that five highly conserved hydrophobic residues create a pocket, or alcove, just above the ACS active site where CO enters and is concentrated, favoring its binding to the active site’s catalytic Ni. There, it is combined with the methyl group and CoA to yield the acetyl-CoA product.
Through the use of in vitro and in vivo growth, kinetic, spectroscopic, and binding experiments, one of the five alcove residues, F229, was found to play a pivotal role in coordinating CO binding to the ACS active site in a size-dependent manner. Mutation of this residue to the much smaller residue alanine was found to decrease the CO affinity of the active site 30-fold, abolishing the ability of the host organism to grow autotrophically on CO2. In contrast, mutation of F229 to the much larger residue tryptophan enhanced CO affinity 80-fold.
The Impact
The alcove formed by the described residues augments and tunes the gas-bonding properties of the active-site Ni2-iron-sulfur metallo-cofactor. Mutation of these residues presents new ways in which the carbon-fixing capabilities of the Wood-Ljungdahl pathway can be enhanced. These residues are now considered integral to ACS functioning to the extent that they should be considered part of the active site, despite not being directly bound to the Ni2-iron-sulfur metallo-cofactor. Similar, yet-undescribed, alcoves likely exist in other gas-handling metalloenzymes. These should be sought out to improve understanding of small-gas metabolism, which underpins all life.
Funding
The work was supported by R37-GM39451 (S. W. R.); R35-GM141758 (S. W. R.); the U.S. Department of Energy, Office of Science (OS), Office of Basic Energy Sciences (BES), Chemical Sciences, Geosciences, and Biosciences Division, Physical Biosciences Program through FWP 100593 (R. S.); and by the “Carbon-Negative Chemical Production Platform” project under the Energy and Carbon Optimized Synthesis for the Bioeconomy (ECOSynBio) program from the US Department of Energy Advanced Research Projects Agency-Energy (ARPA-E) Funding Opportunity Number DE-FOA-0002387. SSRL support is also acknowledged from the DOE OS, Office of Biological and Environmental Research; and by the NIH NIGMS (SSRL SMB Program). Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Institute of General Medical Sciences (P30GM133894).
Related Links
References
Wiley, S., Griffith, C., Eckert, P., Mueller, A. P., Nogle, R., Simpson, S. D., Köpke, M., Can, M., Sarangi, R., Kubarych, K., and Ragsdale, S. W. 2024. “An Alcove at the Acetyl-CoA Synthase Nickel Active Site is Required for Productive Substrate CO Binding and Anaerobic Carbon Fixation,” Journal of Biological Chemistry 300(8), 107503. DOI: 10.1016/j.jbc.2024.107503
