Molecular Basis of Alternative Tyrosine Biosynthesis in Plants
06/26/2017
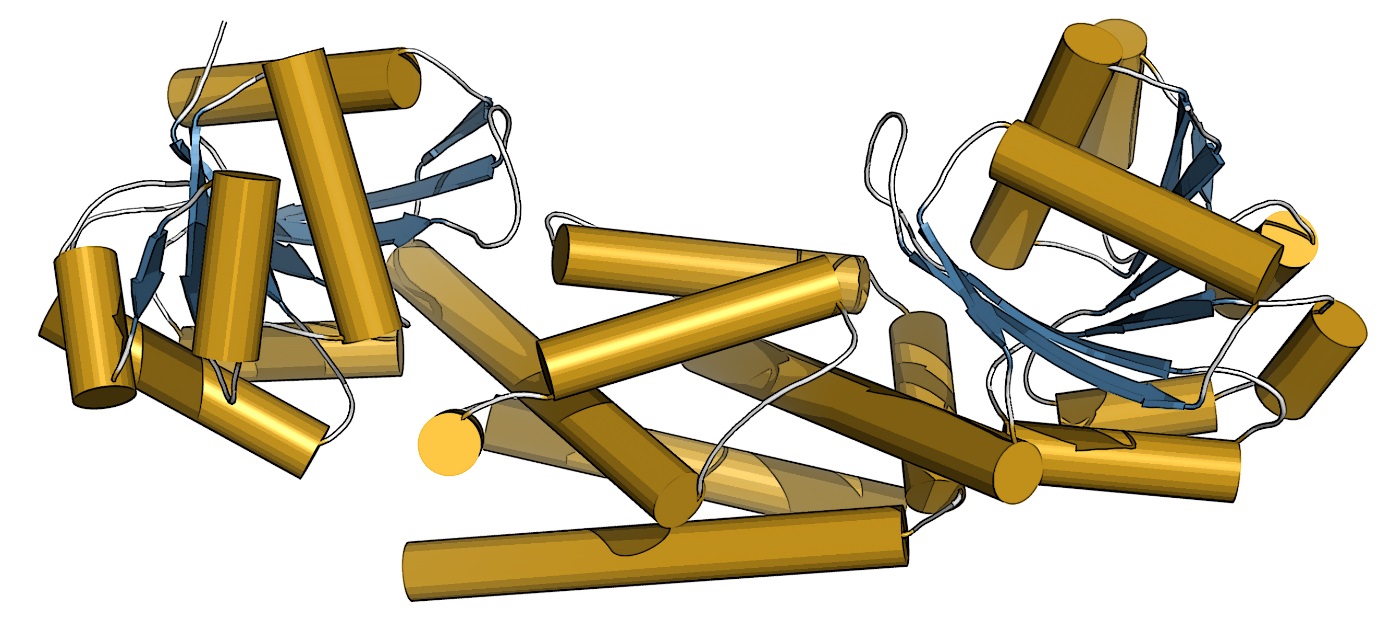
The three-dimensional structure of the prephenate dehydrogenase (PDH) enzyme from soybean. This structure helped show that only one mutation in PDH allowed legumes to evolve a new way to make the amino acid tyrosine. [Courtesy Cynthia Holland, Washington University in St. Louis.]
The Summary
Peanuts have not one, but two ways to make the amino acid tyrosine, an essential plant and human nutrient. Using X-ray crystallography, scientists discovered a mutated form of a key enzyme plants used to make tyrosine. In cherry tomatoes, the basic canonical form of the enzyme dominates; peanuts can switch hit; and some strains of soybeans have lost the canonical form. The discovery demonstrates that primary metabolism can evolve.
Researchers have tied this major evolutionary change in plant metabolism to a single mutation in the new enzyme. The more recently evolved of the two pathways for making tyrosine is much less constrained than the earlier one, raising the possibility of using it in commercial production of drugs or nutrients at high yields.
Instruments and Facilities
X-ray macromolecular crystallography; diffraction data collected at beamline 19-ID of the Advanced Photon Source at the Argonne National Laboratory Structural Biology Center.
Funding
Work supported by the National Science Foundation (NSF; IOS-1354971 to H.A.M. and MCB-1614539 to J.M.J.). C.K.H. support: NSF Graduate Research Fellowship Program (DGE-1143954). Portions of research carried out at Argonne National Laboratory (ANL) Structural Biology Center (SBC) of the Advanced Photon Source (APS), a national user facility operated by the University of Chicago for the Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE) Office of Science (DE-AC02-06CH11357).
Related Links
- BER Resource: Structural Biology Center
- Feature Story: Pulling the tablecloth out from under essential metabolism
- News: Peanut family secret for making chemical building blocks revealed
References
Schenck, C. A., et al. “Molecular Basis of the Evolution of Alternative Tyrosine Biosynthetic Routes in Plants.” Nat. Chem. Biol. 13, 1029–1035 (2017). [DOI:10.1038/nchembio.2414].
