Multifunctionality of an Ebola Virus Protein
08/15/2013
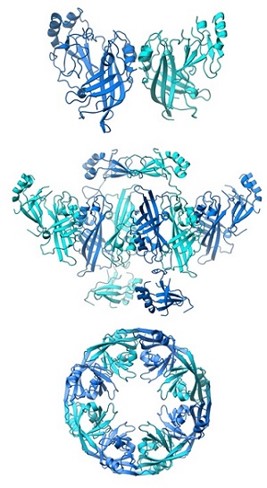
The Ebola virus VP40 protein assumes different structures to perform multiple roles in the virus’s life cycle. Top, a butterfly-shaped dimer structure critical for membrane trafficking. Middle, a rearranged hexameric structure essential for building and releasing nascent virions. Bottom, an RNA-binding octameric ring that controls transcription in infected cells. [Image credit: C. Corbaci, Z. Bornholdt, E. Ollmann Saphire, The Scripps Research Institute.]
Proteins, particularly viral proteins, can be multifunctional, but the mechanisms behind multifunctionality are not fully understood. Scientists illustrated through multiple crystal structures, biochemistry, and cellular microscopy that the Ebola virus protein VP40 rearranges into different structures, each with a distinct function required for the ebolavirus life cycle.
A butterfly-shaped VP40 dimer traffics to the cellular membrane. Once there, electrostatic interactions trigger rearrangement of the polypeptide into a linear hexamer. These hexamers construct a multilayered, filamentous matrix structure that is critical for budding and resembles tomograms of authentic virions. A third structure of VP40, formed by a different rearrangement, is not involved in virus assembly but instead uniquely binds RNA to regulate viral transcription inside infected cells.
These results provide a functional model for ebolavirus matrix assembly and the other roles of VP40 in the virus life cycle and demonstrate how a single wild-type, unmodified polypeptide can assemble into different structures for different functions.
Funding Acknowledgements
Beamlines at Argonne National Laboratory’s (ANL) Advanced Photon Source (APS): 19-ID and GM/CA 23-ID; SLAC National Accelerator Laboratory’s (SLAC) Stanford Synchrotron Radiation Lightsource (SSRL): 12-2; and Lawrence Berkeley National Laboratory’s (LBNL) Advanced Light Source (ALS) 5.0.2. E.O.S. support: Career Award in the Biomedical Sciences and an Investigator in Pathogenesis of Infectious Disease Award from Burroughs Welcome Fund, as well as The Skaggs Institute of Chemical Biology and a National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Disease (NIAID) award (R43 AI1088843). Z.A.B. support: grant (2T32AI007244) to The Scripps Research Institute (TSRI; manuscript #21649) Department of Immunology and Microbial Science. Y.K.: membership within and support from the Region V “Great Lakes” Regional Center for Excellence (RCE) for Biodefense and Emerging Infectious Disease Research Program (NIH award U54 AI057153). T.N. support: Grant-in-Aid for Young Scientists from Japan Society for the Promotion of Science and Grant-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science, and Technology.
Related Links
- BER Resource: Structural Biology Center
- BER Resource: Structural Molecular Biology Resource
- Feature Story: Structural rearrangement in Ebola virus protein VP40 creates multiple functions
- News: Team reveals how deadly Ebola virus assembles
- News: Lethal viral shape-shifter
References
Bornholdt, T. Noda, D.M. Abelson, P. Halfmann, M.R. Wood, Y. Kawaoka, E. Ollmann Saphire. “Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle,” Cell 154, 763 (2013). [DOI: 10.1016/j.cell.2013.07.015].
