Chemical Strategy for Conversion of CO2 to Biomass
08/03/2017
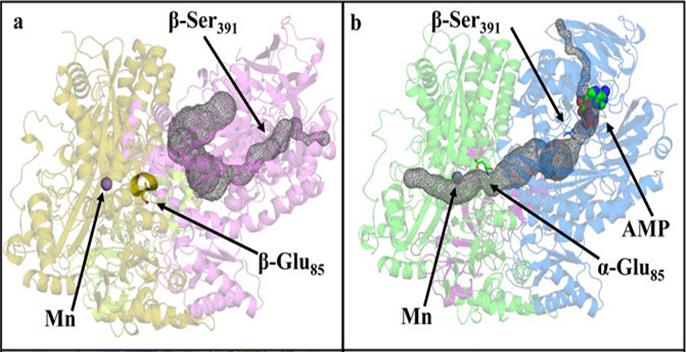
Upon nucleotide binding, acetone carboxylase undergoes conformational shifts, opening an internal solvent channel. (a) Ligand-free structure showing a substrate channel (grey) linking the nucleotide binding site to the outside solvent and allowing adenosine triphosphate (ATP) cofactor and carbon dioxide (CO2) substrate to enter. Access to the manganese (Mn) active site is closed by an a-helix in the path. (b) The adenosine monophosphate (AMP)–bound structure shows an opening of an internal channel (grey) linking the nucleotide binding site to the Mn active site. The blocking helix becomes a disordered loop when AMP is bound. [From Mus, F., et al. 2017. DOI:10.1038/s41598-017-06973-8. Reused under a Creative Commons license (CC by 4.0, https://creativecommons.org/licenses/by/4.0/).]
The Summary
A research team has uncovered a new mechanism for enzyme-mediated carbon dioxide (CO2) capture and conversion. They have revealed new and unique elements of carboxylation chemistry (CC) that could be used in catalytic strategies for converting CO2 into chemical feedstock or biomass.
The crystal structure of acetone carboxylase (AC; enzyme involved in biodegradation by bacteria) was solved using X-ray crystallographic data. The inactive, unbound (apo) structure shows a substrate channel blocked off from the manganese (Mn) active site where CO2 conversion takes place. An adenosine monophosphate (AMP)–bound structure contains large conformational changes that open the channel to the active site. Highly reactive intermediates in the channel are protected from outside solvent as they are transported to the Mn active site for CO2 conversion.
Stepwise mechanisms of carboxylation reactions differ in essential ways with respect to co-substrate, co-factor, and metal requirements. Knowledge of these mechanisms provides the basis for an increased fundamental understanding of CC, contributing to future strategies for CO2 capture and conversion to biomass. These in turn may mitigate the effects of increasing concentrations of CO2 on the global climate.
Instruments and Facilities
X-ray crystallographic data from the Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC National Accelerator Laboratory (SLAC). Structural data from X-ray macromolecular crystallography were measured on SSRL beamline 12-2; Advanced Photon Source (APS) at Argonne National Laboratory (ANL); and the Diamond Light Source, Oxfordshire, United Kingdom.
Funding
Work supported by the Office of Basic Energy Sciences (OBES), U.S. Department of Energy (DOE) Office of Science, under Award Number DE-FG02-04ER15563. The Stanford Synchrotron Radiation Lightsource (SSRL) Structural Molecular Biology Program at the SLAC National Accelerator Laboratory (SLAC) supported by the DOE Office of Biological and Environmental Research (OBER) and by the National Institutes of Health’s (NIH) National Institute of General Medical Sciences (NIGMS; including P41GM103393). Argonne National Laboratory’s (ANL) Structural Biology Center at the Advanced Photon Source (APS). ANL is operated by University of Chicago Argonne, LLC, for DOE OBER under contract DE-AC02-06CH11357.
Related Links
References
Mus, F., et al. “Structural Basis for the Mechanism of ATP-Dependent Acetone Carboxylation.” Nat. Sci. Rep. 7, Article 7234 (2017). [DOI:10.1038/s41598-017-06973-8].
