Novel Cell Membrane Model Could Be Key to Uncovering New Protein Properties
06/23/2020
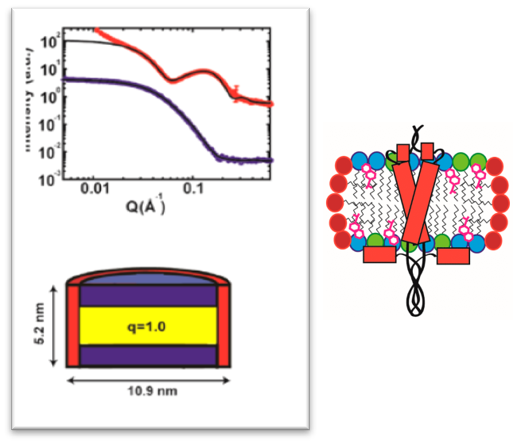
SANS/SAXS data demonstrating the raft-like bicelle structure (left). A cartoon shows new C99 protein structure in the bicelle (right). [Reprinted with permission from Hutchison, J. M., et al. 2020. “Bicelles Rich in both Sphingolipids and Cholesterol and Their Use in Studies of Membrane Proteins,” J. Am. Chem. Soc. 142(29), 12715–729. DOI:10.1021/jacs.0c04669. Copyright 2020 American Chemical Society.]
Scientific Achievement
Bicelles rich in sphingomyelin and cholesterol have been developed to mimic raft-like membranes for membrane protein research. Human amyloid precursor protein C99 in the raft-like bicelles is found to be low-order oligomer in contrast to previous studies.
Significance and Impact
Raft-like bicelles developed in this study provide a novel model membrane system for membrane proteins that require sphingomyelin and cholesterol for proper structure and function.
Research Details
- Small-angle Neutron and X-ray Scattering were used to characterize the bicelle morphology, demonstrating the monodisperse, bilayer structure and sphingomyelin/cholesterol integration in the membrane.
- An NMR study of C99 protein found a new oligomerization state in the raft-like membrane bicelles compared to previous reports.
Related Links
- BER Resource: Center for BioMolecular Structure
- BER Resource: Center for Structural Molecular Biology
- Feature Story: Novel Cell Membrane Model Could Be Key to Uncovering New Protein Properties
References
James M. Hutchison, et al. Bicelles Rich in both Sphingolipids and Cholesterol and Their Use in Studies of Membrane Proteins. J. Am. Chem. Soc. 2020, 142, 29, 12715–12729. DOI: 10.1021/jacs.0c04669
