Persistence and Mobility of Iron-Rich Colloids Facilitate Element Transport and Cycling
06/30/2023
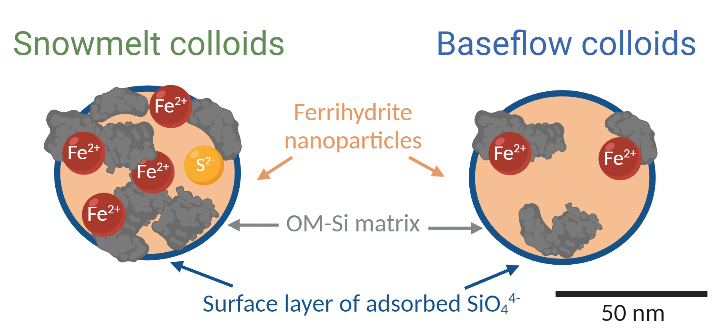
Characterization of Iron-Rich Colloids from an Anoxic Riparian Zone Explains their Persistence and Impacts on Iron and Nutrient Transport in Watersheds. Soil water samples from riparian anoxic zones of a redox-active floodplain across the hydrological transition from snowmelt to baseflow conditions serve as a model for river-floodplain systems in Alpine environments. The colloids were identified as multi-phase nanoparticles comprised of ferrihydrite coated by Si and OM that is further associated with Fe(II) and Fe(III). The colloids exhibited a seasonally variable composition, with a lower contribution of Fe(II) under more oxic baseflow conditions.
[Environmental Molecular Sciences Laboratory]
The Science
Subsurface interfaces are ubiquitous in floodplain environments and sustain a multitude of biogeochemical processes, including the formation and release of reactive, mobile colloids. Colloids are known vectors of micronutrient, contaminant, and organic matter transport and are suspected to be important export agents from floodplains to stream water. Iron (Fe)-rich mobile colloids play vital yet poorly understood roles in the biogeochemical cycling of Fe in groundwater by influencing organic matter (OM) preservation and fluxes of Fe, OM, and essential micronutrients.
Researchers from the SLAC National Accelerator Laboratory, the Environmental Molecular Sciences Laboratory (EMSL), and other institutions detected Fe-rich colloids in anoxic groundwater of a redox-active floodplain near Slate River, Colo, where the colloids accounted for up to 72% of aqueous Fe. They characterized the colloids using a wide array of techniques including cryo-transmission electron microscopy (cryo-TEM), TEM-energy dispersive spectroscopy (TEM-EDS), Mössbauer spectroscopy, and Fe-extended X-ray absorption fine structure (Fe-EXAFS). The colloids comprise mixed-phase assemblages of ferrihydrite nanoparticles coated with silicon and enmeshed in organic matter. Both Fe(II) and Fe(III) co-existed in the colloids under anoxic conditions, illustrating the passivating (i.e., corrosion resistant) effects of the silicon and organic matter matrix against redox-triggered transformations.
The Impact
The ability of ferrihydrite-based colloids to withstand anoxic conditions rich in dissolved Fe(II) highlights the extent to which silicon and organic matter coatings can protect Fe(III) from reductive dissolution. This passivating (i.e., corrosion deterring) feature may also explain the existence of Fe(II) and sulfur within the colloidal structure.
Ultimately, the persistence of the colloids suggests they may transport throughout anoxic zones and reach oxic surface waters. The findings shed light on the composition and dynamics of natural Fe-rich colloids in floodplain systems, with implications to elemental transport and cycling.
The Summary
Researchers used a combination of advanced characterization techniques to decipher the composition of Fe-rich colloids at a floodplain field site near Slate River, Colo. Cascade filtering revealed the presence of Fe-rich colloids in the riparian anoxic soil water and their abundance and composition varying with season. Cryo-EM and TEM-EDS imaging showed monodispersed and nanoassemblages of spherical colloids in the 10-50 nm range containing Fe, oxygen, silicon, carbon, and calcium. TEM selected-area electron diffraction analysis and Mössbauer spectroscopy indicated a poorly crystalline ferrihydrite-like phase. Fe-EXAFS further verified ferrihydrite and Fe(II)- and Fe(III)-organic matter interactions, as well as a small contribution from Fe-sulfur bonding.
The analyses indicate that the colloids were primarily composed of nanosized ferrihydrite spheres stabilized by organic matter, silicon, and bridging cations such as calcium. The persistence of Fe(III)-rich colloids in primarily anoxic zones suggests that the silicon-organic matter coating serves as a passivating layer against reduction, but its efficiency likely depends on biogeochemical and hydrological conditions.
Funding
Funding was provided by the U.S. Department of Energy (DOE) office of Biological and Environmental Research (BER), Environmental System Science Division, through its support of the SLAC Floodplain Hydro-Biogeochemistry Science Focus Area under Contract No. DE- AC02–76SF00515. The Stanford Synchrotron Radiation Lightsource (SSRL) and SLAC are supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences. The technical staff at SSRL are gratefully acknowledged for their support of the X-ray absorption measurements. We further acknowledge the use and support of the Stanford-SLAC Cryo-EM Facilities, which is supported by the National Institutes of Health Common Fund Transformative High-Resolution Cryo-Electron Microscopy program (U24 GM129541). W.Z is funded by the Stanford Interdisciplinary Graduate Fellowship program. A portion of this research was performed on a project award (10.46936/ lser.proj.2021.51929/60000375) from the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility sponsored by the Biological and Environmental Research program under Contract No. DE-AC05–76RL01830.
Related Links
References
Engel, M., et al. 2023. “Structure and Composition of Natural Ferrihydrite Nanocolloids in Anoxic Groundwater,” Water Research, 238 (119990). DOI:10.1016/j.watres.2023.119990.
