Potentially Customizable Molecular Assembly Line
11/04/2021
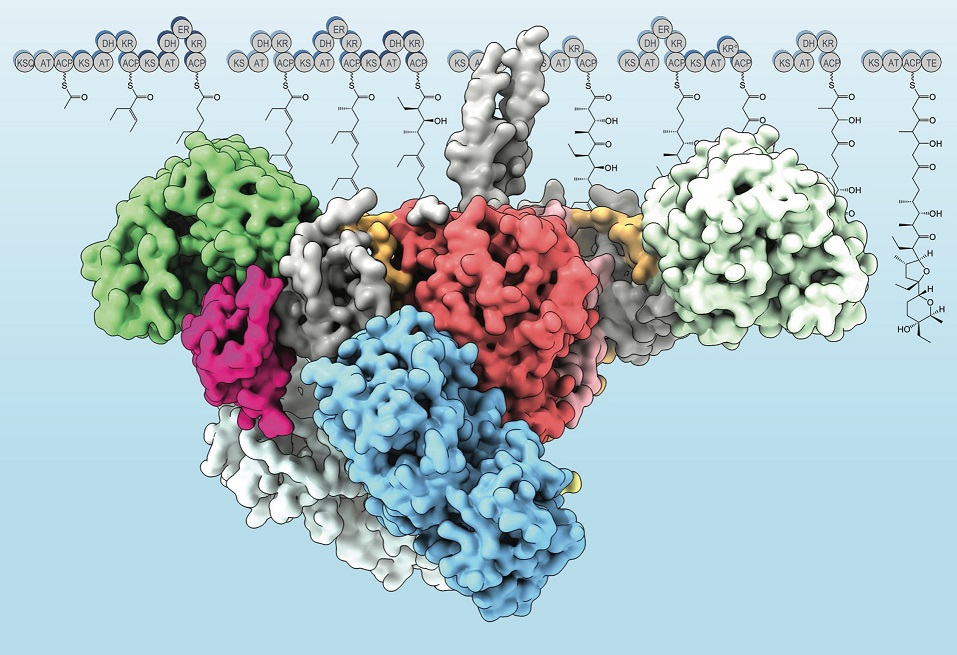
A model of the modular polyketide synthase Lsd14 enzyme derived from X-ray crystallography. In two papers, researchers studied the structure and assembly-line mechanism of molecules that help build common antibiotics. [Image courtesy of Saket Bagde.]
Researchers used X-ray macromolecular crystallography (MX) and cryogenic electron microscopy (cryo-EM) to describe the atomic structure of a modular enzyme—a polyketide synthase—intact and at different stages of its reaction cycle. The experiments revealed that the enzyme, a molecular assembly line producing common antibiotics, is composed of two interacting yet asynchronously-operating reaction chambers.
A high-resolution structure of an intact polyketide synthase module had remained elusive for over 30 years since the enzyme’s discovery. The team used multiple beamlines at the Stanford Synchrotron Radiation Lightsource, the Advanced Light Source, and the Advanced Photon Source over a period of three years to elucidate its structure and mechanism. The unprecedented detail will inform future studies aimed at engineering polyketide synthases to produce “green” antibiotics, pharmaceuticals, and novel biofuels.
Related Links
- BER Resource: Structural Molecular Biology Resource
- Feature Story: Researchers probe secrets of natural antibiotic assembly lines
- Feature Story: Enzyme research unlocks gateway for new medicines
References
Bagde, S. R.; Mathews, I. I.; Fromme, J. C.; Kim, C.-Y. 2021. Modular polyketide synthase contains two reaction chambers that operate asynchronously. Science, 374 (6568), 723-729. [DOI: 10.1126/science.abi8532]
Cogan, D.P., et al. 2021. Mapping the catalytic conformations of an assembly-line polyketide synthase module. Science, 374 (6568), 729-734. [DOI: 10.1126/science.abi8358]
