Probing S-layer Protein Structural Dynamics with SAXS
05/09/2017
Calcium mediates the structural state of the Caulobacter crescentus surface layer protein, RsaA. [Image featured on the cover of Biophysical Journal Volume 112, Issue 9, 2017. [CC BY-NC-ND 4.0]]
The Summary
All archaea and many bacteria possess a protein shell referred to as a surface layer (S-layer), which usually consists of a single protein self-assembled into a two-dimensional (2D) crystal lattice. Studies have revealed the structural dynamics of this S-layer protein from the model bacterium Caulobacter crescentus, called RsaA.
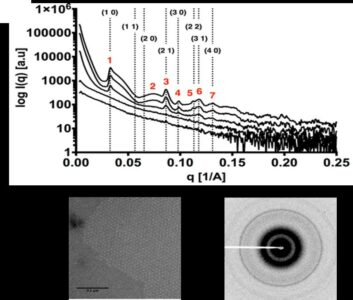
Small angle X-ray scattering and diffraction (SAXS/D) data of five solutions with different concentrations of the Caulobacter crescentus S-layer protein, RsaA, in the presence of calcium (Ca). Scattering profiles indicate concentration-dependent crystallization. Automatic indexing of the numbered peaks yielded a hexagonal crystal lattice consistent with predictions and denoted by Miller indices. (Top) The diffraction pattern obtained for the highest concentration used (8 mg/ml) shows powder rings. (Bottom) Transmission electron microscopy of the 8 mg/ml RsaA in the presence of 10 millimole per Liter (mm/L) of calcium chloride (CaCl2) (scale bar 200 nm). [Reprinted from Herrmann, J., et al. (2017). DOI:10.1016/j.bpj.2017.04.003. Copyright 2017, with permission from Elsevier.]
Enabling differentiation of the discrete states, these results rationalize physiological data implicating RsaA as a player in environmental adaptation of C. crescentus. The findings also provide a biochemical and physiological basis for RsaA’s calcium (Ca)-binding behavior, which extends far beyond Ca’s usual role in S-layer biology of aiding biogenesis or oligomerization, and demonstrate a connection to cellular fitness. Further characterization using slow and fast time-resolved SAXS/D methods is ongoing.
Instruments and Facilities
Stanford ChEM-H Macromolecular Structure Knowledge Center, Stanford Department of Structural Biology Electron Microscopy Center, Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC National Accelerator Laboratory (SLAC). Beamlines or instruments used: transmission electron microscopy (TEM) and small angle X-ray scattering and diffraction (SAXS/D) at SSRL beamline 4-2 at SLAC.
Funding
Part of work performed at Stanford University’s ChEM-H Macromolecular Structure Knowledge Center and Department of Structural Biology Electron Microscopy Center. Support: U.S. Department of Energy (DOE), SLAC National Accelerator Laboratory (SLAC) Laboratory Directed Research and Development (co-PI: John Bargar), under contract No. DE-AC02-76SF00515. Material based on work supported by the Office of Biological and Environmental Research (0BER) Mesoscale to Molecules: Bioimaging Science Program, DOE Office of Science. J.H. support: National Science Foundation (NSF) Graduate Research Fellowship Program (NSF-GRFP) and DOE Office of Science Graduate Student Research Program (DOE-SCGSR). J.S. support: grant from Natural Sciences and Engineering Research Council of Canada. L.S. support: National Institutes of Health’s (NIH) National Institute of General Medical Sciences (NIGMS; R35118072A). Use of Stanford Synchrotron Radiation Lightsource (SSRL), SLAC, support: Office of Basic Energy Sciences (OBES), DOE Office of Science, under contract No. DE-AC02-76SF00515. SSRL Structural Molecular Biology Program support: DOE OBER and NIH NGMIS (including grant No. P41GM103393).
Related Links
References
Herrmann, J., et al. “Environmental Calcium Controls Alternate Physical States of the Caulobacter Surface Layer,” Biophys. J. 112(9), 1841–1851 (2017). [DOI:10.1016/j.bpj.2017.04.003].
