Role of Enigmatic “Red Body” in a Biofuel-Candidate Alga
06/27/2024
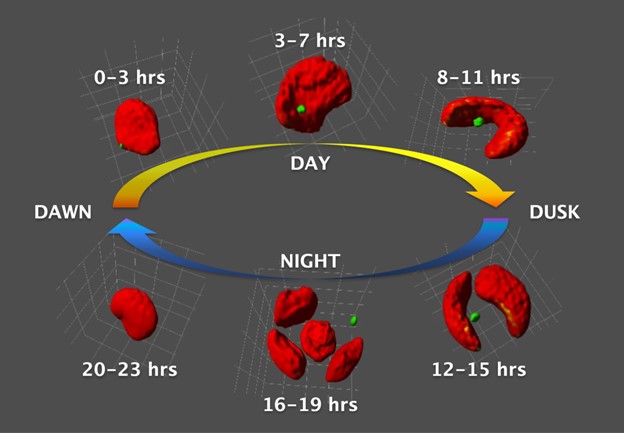
Fourier Transform Infrared Spectroscopy Helps Reveal the Role of Mysterious Red Organelles. “Red body” organelles, colored green in this figure, transport biopolymer molecules to the cell walls of the alga Nannochloropsis oceanica, supporting the rapid growth of new daughter cells. Three-dimensional reconstructions of the algal cells (colored red in this figure; not to be confused with the green-colored “red bodies”) were created from super-resolution two-dimensional z-stacks. The reconstructions reveal the development and release of the green “red body,” which coincides with the alga’s 24-hour life cycle. Note: each reconstruction is bound by a 4-micron cubic volume element to ensure comparable scale.
[Courtesy Elizabeth Boatman and Hoi-Ying Holman, Advanced Light Source]
The Science
Scientists have long searched for new ways to make fuel and have consequently studied algae that naturally produce and retain large amounts of fatty molecules. Yet questions remain regarding the basic life cycles of algal species of interest, such as Nannochloropsis oceanica and other Eustigmatophytes. The marine nanophytoplankton N. oceanica possess an enigmatic structural feature called the “red body.” Now, Fourier transform infrared spectroscopy has revealed that the globular red body contains an accumulation of antioxidant carotenoids, responsible for the red color, and large quantities of long-chain aliphatic lipids, a type of fatty molecule.
In the same study, ultra-performance liquid chromatography coupled with high-resolution mass spectrometry detected a C32 alkyl diol, a potential precursor of the material algaenan, which is a recalcitrant cell wall polymer produced by some green algae. Transmission electron microscope imaging and 3D cryo-tomography indicated the red body is a membrane-bound organelle that likely facilitates the transport of key molecules needed for cell wall construction, supporting the ability of N. oceanica to rapidly divide into two, four, or even eight daughter cells every 24 hours. Without the organelle, N. oceanica could face challenges transporting the hydrophobic molecules, which include the C32 alkyl diol, through its water-based interior.
This study involved collaboration among researchers from the University of California-Berkeley, the Lawrence Berkeley National Laboratory (LBNL), and the University of Copenhagen in Denmark. LBNL is home to the Berkeley Synchrotron Infrared Structural Biology (BSISB) program, funded by DOE’s Biological and Environmental Research program, and the Advanced Light Source, a DOE Office of Science user facility.
The Impact
As the effects of climate change continue to grow, scientists face mounting calls to deliver alternative fuels with carbon-neutral emissions when burned. Eustigmatophytes, a group of single-celled algae found in freshwater, marine, and terrestrial environments, could offer an opportunity to produce new biofuels.
However, a limited understanding of the life cycle and cell biology of the model species N. oceanica has restricted researchers’ ability to draw conclusions that could otherwise inform cultivation and genetic engineering directions of additional algae species considered to be good candidates for producing biofuels and/or sequestering carbon. This work represents a new biological link between molecular and large-scale processes in cellular systems.
The Summary
Because few algae species exhibit the red body characteristic of N. oceanica, its role in the organism’s cellular life cycle has remained largely unknown. This pigmented organelle initially forms adjacent to the cell’s food-producing plastid before ultimately being released outside the cell wall during autosporangial division. A parent N. oceanica alga divides into daughter cells via spore production in a process known as autospore release.
Scientists’ interest in Eustigmatophyte algae is two-fold: its rapid growth and its ability to partition up to half its mass into valuable lipids. These hallmark features indicate the algae’s potential for use in biofuel applications. A range of reference genomes have been published as interest has grown in the potential application of gene editing tools to Eustigmatophyte algae toward biofuel applications.
Once thought rare, Eustigmatophytes have been found in a range of freshwater, marine, and terrestrial systems. Two genera of Eustigmatophyte, Nannochloropsis and Microchloropsis, have been established as model systems. In both groups, cells are solitary, non-motile, and round, with diameters on the order of 2 to 4 microns. Species belonging to both genera reproduce on a diurnal basis via asexual fission, growing during the day and splitting each night.
The presence of a red-orange globule outside the cell’s chloroplast during the daytime part of the life cycle is an identifying feature of Eustigmatophytes. Although the globule has been widely reported throughout the group, this work provides the first account of its formation and biological function.
Imaging techniques included ultra-performance liquid chromatography coupled with high-resolution mass spectrometry, various laser and electron microscopy methods, and Fourier transform infrared spectroscopy. The intriguing autofluorescent, globular nature of the red body — with its distinct compartmentalization and differentiation — led researchers to define it as a membrane-bound organelle.
Further study of the red body’s contents led researchers to hypothesize that it could be a delivery vessel for molecules used in cell wall construction. During the daytime portion of the life cycle, N. oceanica cells grow rapidly. At night, each cell divides into multiple daughter autospores. With the production of multiple daughter cells instead of just one, significantly more cell wall material is needed to fully encapsulate each new cell.
The red body aids the encapsulation process, researchers believe, by making available large amounts of material to make a specific part of the cell wall known as algaenan. Infrared spectroscopy analyses back up this hypothesis, revealing that red bodies discarded after autospore generation contain a range of precursor and intermediate products needed for cell wall formation, in addition to some fully polymerized algaenan matter.
Researchers see N. oceanica as a model organism for understanding the biosynthesis of “chemically recalcitrant lipidic biopolymers” via plastid-derived fatty molecules that must be transported through an aqueous inner-cell environment to the cell wall. Similar molecules are ubiquitous throughout plant lineages because they play a key role in plant physiology by controlling the movement of water within and around plant bodies and the cells that constitute them. Until now, many details related to the transport of these molecules within the cell, and their final polymerization process during cell wall construction, remained unknown. As a result, this work contributes new insight into the biological link between molecular and large-scale processes at the cellular level.
Related Links
- BER Resource: Berkeley Synchrotron Infrared Structural Biology Imaging Program
- BSISB Highlight: Scientists Unlock Secrets of Mysterious 'Red Body’ in a Biofuel-Candidate Alga
References
Gee, C.W., Andersen-Ranberg, J., Boynton, E. et al. 2024. “Implicating the Red Body of Nannochloropsis in Forming the Recalcitrant Cell Wall Polymer Algaenan,” Nature Communications 15, 5456. DOI:10.1038/s41467-024-49277-y.
