SAXS Elucidates a Microbial Bioenergetic Pathway
11/27/2023
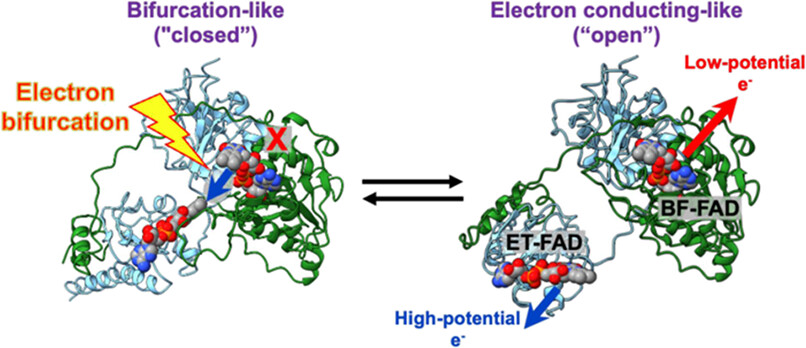
A schematic shows the metabolic process of electron bifurcation in the anaerobic bacterium Thermotoga maritima. The pictured enzyme, Fix/EtfABCX, sends one electron to an acceptor with a high reduction potential and another to an acceptor with a lower reduction potential. Coupling these two redox reactions leverages the thermodynamically favorable reaction to support the unfavorable one.
[Reprinted under a Creative Commons license (CC BY 4.0) from Murray et al. 2024. DOI:10.1021/acs.biochem.3c00472]
The Science
Electron bifurcation, a class of chemical reaction found only in biology, liberates two electrons from one donor and channels them to two separate electron acceptors. Analogous to a mechanical pulley system in which one weight rises as the other falls, one electron is elevated in energy at the expense of lowering the energy of the second. The higher-energy electron can then help perform high-energy work, such as bond-breaking or bond-making, as necessary for the organism’s survival.
Now, a team of researchers from the Lawrence Berkeley National Laboratory and other institutions has used small-angle x-ray scattering (SAXS) at the Advanced Light Source (ALS) to understand an important protein involved in this bioenergetic pathway—the EtfABCX protein from the anaerobic bacterium Thermotoga maritima. In experiments performed at ALS Beamline 12.3.1 (SIBYLS), the team studied the mechanism of electron channeling in the bifurcation process. SAXS, a solution-based technique that does not restrain motion, enabled researchers to elucidate the steps, triggers, and coordinated conformational changes of EtfABCX. The results suggest that the protein couples electron transfer to conformational change.
The Impact
By following the mechanics and conformations of EtfABCX, the researchers were able to shed light on this novel biological mechanism where energetic electrons are channeled over relatively large distances. Understanding these novel bioenergetic systems will help build microbial energy flux models and aid in the annotation of newly discovered genes.
Funding
A portion of this work was conducted at the Advanced Light Source (ALS), a national user facility operated by Lawrence Berkeley National Laboratory on behalf of the Department of Energy, Office of Biological and Environmental Research through the Integrated Diffraction Analysis Technologies (IDAT) program. Additional support comes from the DOE BES award “Hyperthermophilic Multiprotein Complexes and Pathways for Energy Conservation and Catalysis” (DE-FG0295-95ER20175), the National Institute of Health project ALS-ENABLE (P30 GM124169), the High-End Instrumentation grant S10OD018483, and the National Institutes of Health grant GM135088.
Related Links
- BER Resource: Structurally Integrated Biology for the Life Sciences
- ALS Science Brief: Bifurcation of High- and Low-Energy Electrons in Microbial Metabolism
- Berkeley Lab News: Researchers Leverage SAXS to Understand Aspect of Microbial Metabolism
References
Murray, D. T., et al. 2024. “Correlating Conformational Equilibria with Catalysis in the Electron Bifurcating EtfABCX of Thermotoga maritima,” Biochemistry 63(1), 128–40. DOI:10.1021/acs.biochem.3c00472
