Biocatalyst Inspiration: Specificity and Selectivity of Rieske Oxygenases
01/11/2022
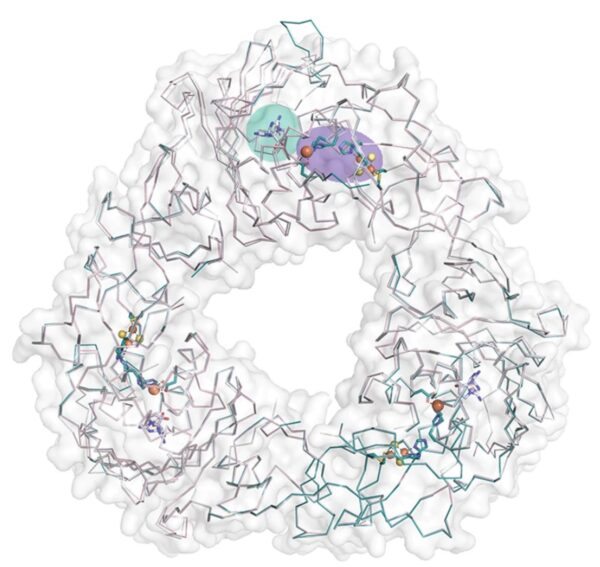
The Rieske oxygenase trimeric structure with bridging Rieske metal centers in purple and the substrate-binding site of one subunit in teal. Mutation variants catalyze the hydroxylation of different carbon atoms on substrates. [Reprinted under a Creative Commons Attribution 4.0 International License (CC BY 4.0) from J. Liu et al. 2022. DOI: 10.1038/s41467-021-27822-3]
The Summary
Rieske non-heme iron oxygenases represent one of nature’s solutions for performing precise site-selective C–H bond functionalization reactions by exploiting the reactivity of iron. Thus far, only a handful of Rieske oxygenases have been structurally characterized and remarkably little information exists regarding how these enzymes use a common architecture and set of metallocenters to facilitate a diverse range of reactions.
To investigate the architectural parameters that dictate the substrate specificity and site-selectivity of a Rieske oxygenase catalyzed reaction, scientists focused on two Rieske oxygenases, SxtT and GxtA, which are involved in the biosynthesis of paralytic shellfish toxins. They used macromolecular X-ray crystallography to detail how SxtT and GxtA use different protein regions to influence the site-selectivity of their catalyzed monohydroxylation reactions and the location of a substrate access tunnel to the active site.
The structural information allowed for the identification of six residues distributed between three regions of SxtT that together control the selectivity of the C–H hydroxylation event. Substitution of the residues produced a SxtT variant fully adapted to exhibit the non-native site-selectivity and substrate scope of GxtA. Importantly, the selectivity regions are conserved in other structurally characterized Rieske oxygenases, providing a framework for predictively repurposing and manipulating Rieske oxygenases as biocatalysts.
Related Links
References
Liu, J.; Tian, J.; Perry, C.; Lukowski, A.L.; Doukov, T. I.; Narayan, A.R.H.; Bridwell-Rabb, J. 2022. Design principles for site-selective hydroxylation by a Rieske oxygenase. Nature Communications, 13, 255. [DOI: 10.1038/s41467-021-27822-3]
