Structural Basis for Neutralization of Betacoronaviruses by Llama Antibodies
06/11/2020
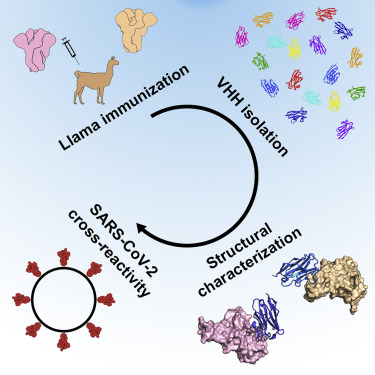
Using llamas immunized with prefusion-stabilized betacoronavirus spike proteins, Wrapp et al. identified neutralizing cross-reactive single-domain camelid antibodies, which may serve not only as useful reagents for researchers studying the viruses causing MERS, SARS, and COVID-19, but also potential therapeutic candidates. Crystal structures further revealed how these antibodies bind spike proteins to prevent virus entry into cells. [Image reprinted from Wrapp et al., 2020, Cell 181, 1004–1015 May 28, 2020. Copyright 2020 Elsevier Inc. https://doi.org/10.1016/j.cell.2020.04.031]
The Summary
Coronaviruses use a large envelope protein called spike (S) to engage host cell receptors and catalyze membrane fusion. S proteins therefore represent a vulnerable target for therapeutics.
Scientists isolated single-domain antibodies (VHHs) from a llama immunized with prefusion-stabilized coronavirus spike proteins. The VHHs neutralized MERS-CoV and SARS-CoV-1 S pseudotyped viruses.
Crystal structures of the VHHs bound to their respective viral targets revealed two distinct epitopes, but both VHHs interfered with receptor binding. There was also cross-reactivity between the SARS-CoV-1 S-directed VHH and SARS-CoV-2 S; the cross-reactive VHH neutralized SARS-CoV-2 S pseudotyped viruses.
The data provide a molecular basis for the neutralization of pathogenic betacoronaviruses by VHHs and suggest that the molecules may serve as useful therapeutics during coronavirus outbreaks.
Related Links
- BER Resource: Structural Biology Center
- Feature Story: Llama Antibodies Could Block Deadly Coronaviruses from Invading Our Cells
References
Daniel Wrapp, et al. “Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies,” Cell 181 (5), 1004-1015 (2020). DOI: 10.1016/j.cell.2020.04.031
