Structural Basis of Bacterial Spore Germination
05/21/2019
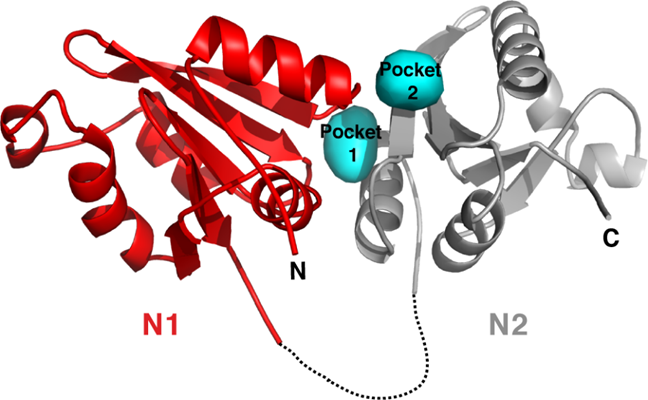
Structural insight into bacterial spore germination could help improve disinfection. The crystal structure of the N-terminal domain of the A subunit of the Bacillus megaterium germinant receptor, GerK3A, reveals the location of two predicted germinant-specific binding pockets (cyan). [Reprinted with permission from Li, Y., et al. 2019. "Structural and functional analyses of the N-terminal domain of the A subunit of a Bacillus megaterium spore germinant receptor." PNAS 116(23), 11470–479. DOI:10.1073/pnas.1903675116.]
Bacterial endospores are found throughout the environment, with some acting as vectors of food spoilage, food poisoning, and infectious diseases. Promoting efficient spore germination could increase spore sensitivity to inactivating measures.
Germination of Bacillus spp. bacteria spores is induced by the interaction of specific nutrient molecules with germinant receptors localized in a spore’s inner membrane. Germinant receptors typically consist of three subunits, referred to as A, B, and C.
Researchers used X-ray macromolecular crystallography to determine the crystal structure of the N-terminal domain (NTD) of the A subunit of the Bacillus megaterium germinant receptor, GerK3. Crystal structure, molecular docking, and biophysical and genetic analyses indicate the presence of a binding pocket at the interface between the two subdomains in the NTD of the A subunit that accommodates specific germinant molecules and plays a critical role in spore germination.
The results can be explored for the development of germinant analogues as either potentiators or inhibitors of spore germination.
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: A Structural Understanding of Spore Germination
References
Li, Y., K. Jin, A. Perez-Valdespino, K. Federkiewicz, A. Davis, M.W. Maciejewski, P. Setlow, and Bing Hao. “Structural and functional analyses of the N-terminal domain of the A subunit of a Bacillus megaterium spore germinant receptor.” PNAS 116 (23), 11470–11479 (2019). [DOI: 10.1073/pnas.1903675116]
