Structure of a Nuclear Membrane Gatekeeper
06/13/2018
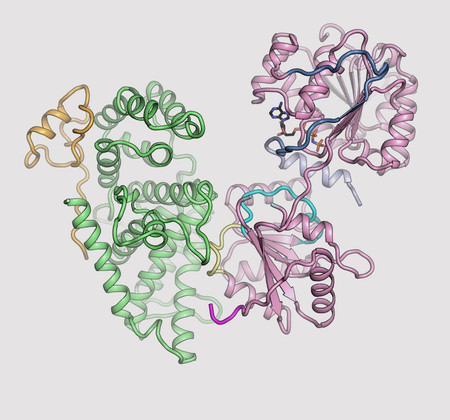
The three-dimensional structures for three proteins in the human nuclear pore complex—Gle1 (green), Nup42 (yellow), and DDX19 (pink)—which together act as gatekeepers for macromolecular transport between the nucleus and cytoplasm. [Reprinted under a Creative Commons Attribution 4.0 International (CC BY 4.0) license from Lin, D. H., et al. 2018. "Structural and functional analysis of mRNA export regulation by the nuclear pore complex." Nature Commun. 9, 2319DOI:10.1038/s41467-018-04459-3.]
The Summary
Nuclear pore complexes (NPC) are macromolecular machines which perforate the nuclear envelope and control passage of molecules such as mRNA from the cell’s nucleus to the cytoplasm. But how NPCs directly participate in macromolecular transport has been poorly understood.
A team of scientists crystalized a protein complex which partially comprises NPCs—Gle1•Nup42—from three different organisms, including humans. Next, they used X-rays from the Frontier Microfocusing Macromolecular Crystallography (FMX) beamline to reveal the 3-D crystal structure for all three organisms, uncovering the evolutionarily conserved binding mode for each. The 3-D structure of the protein complex in humans showed how it ensures mRNA transport. Lastly, they found that Gle1 mutations associated with motor neuron diseases possess severe thermostability defects, suggesting that nucleoporin misfolding contributes to disease.
The results provide the foundation for further mechanistic analyses of mRNA export in humans.
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: Structure of a Nuclear Pore Complex's "Ticket System"
References
D. H. Lin, A. R. Correia, S. W. Cai, F. M. Huber, C. A. Jette, A. Hoelz. Structural and functional analysis of mRNA export regulation by the nuclear pore complex. Nature Commun. 9, 2319 (2018). [DOI: 10.1038/s41467-018-04459-3]
