The Copper Key to More Efficient Biomass Breakdown
11/02/2022
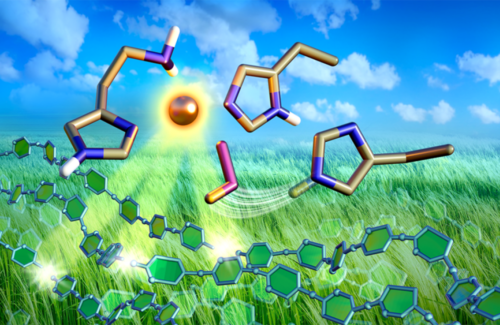
Neutron scattering experiments at the Spallation Neutron Source revealed how the dynamics between copper and oxygen make a special type of enzyme excel at breaking down biomass. Insights could lead to lowering the cost of biofuel production.
[Credit: Jill Hemman/ORNL, U.S. Dept. of Energy]
The Science
Nonfood, plant-based biofuels have potential as sustainable alternatives to fossil fuels, but the enzymes required for production are often too inefficient and costly to produce. However, new research shines a light on fungal enzymes called lytic polysaccharide monooxygenases (LPMOs) that could improve the economic viability of biofuels.
LPMOs excel at breaking down organic matter, but their mechanism of action is unclear. Typical enzymes are made of carbon, nitrogen, hydrogen, and oxygen, but LPMOs also contain copper.
Researchers had previously used neutron scattering at the Spallation Neutron Source and High Flux Isotope Reactor to show how LPMOs bind oxygen to copper to break down biomass. Going a step further, they’ve now used neutron protein crystallography to directly visualize protonation states in the initial steps of oxygen activation directly following active site copper reduction in LPMO9D from the fungus Neurospora crassa. The experiments reveal that the process is driven by an amino acid that donates protons to the oxygen molecule.
The Impact
Understanding the mechanism of LPMOs could enable redesign and testing of different versions with improved efficiency for cellulosic ethanol production.
Funding
Protein expression, purification, and crystallization experiments were conducted at the Center for Structural Molecular Biology (CSMB), a U.S. Department of Energy Biological and Environmental Research User Facility at Oak Ridge National Laboratory. Neutron diffraction data was collected at BL-11B MaNDi at the Spallation Neutron Source (SNS) at ORNL which is sponsored by the Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy. The authors thank Brendan Sullivan for assistance with data reduction. X-ray diffraction data was collected at the Molecular Education, Technology, and Research Innovation Center (METRIC) facilities at North Carolina State University, which is supported by the State of North Carolina. GCS acknowledges support in part from the National Research Foundation (NRF), South Africa and the Oak Ridge National Laboratory Graduate Opportunities (GO!) program. F. M. acknowledges support from USDA NIFA Hatch 211001. This research was supported by the National Institute of General Medical Science R01-GM105978 to P. K. A.
Related Links
- ORNL News: The copper key to more efficient biomass breakdown
- Neutrons run enzyme’s reactivity for better biofuel production
References
Schröder, G.C., et al. 2022. “Capture of Activated Dioxygen Intermediates at the Copper-Active Site of a Lytic Polysaccharide Monooxygenase,” Chemical Science 13, 13303. DOI:10.1039/D2SC05031E.
