Visualizing an Ancient Carbon Cycle Pathway Mechanism
06/12/2023
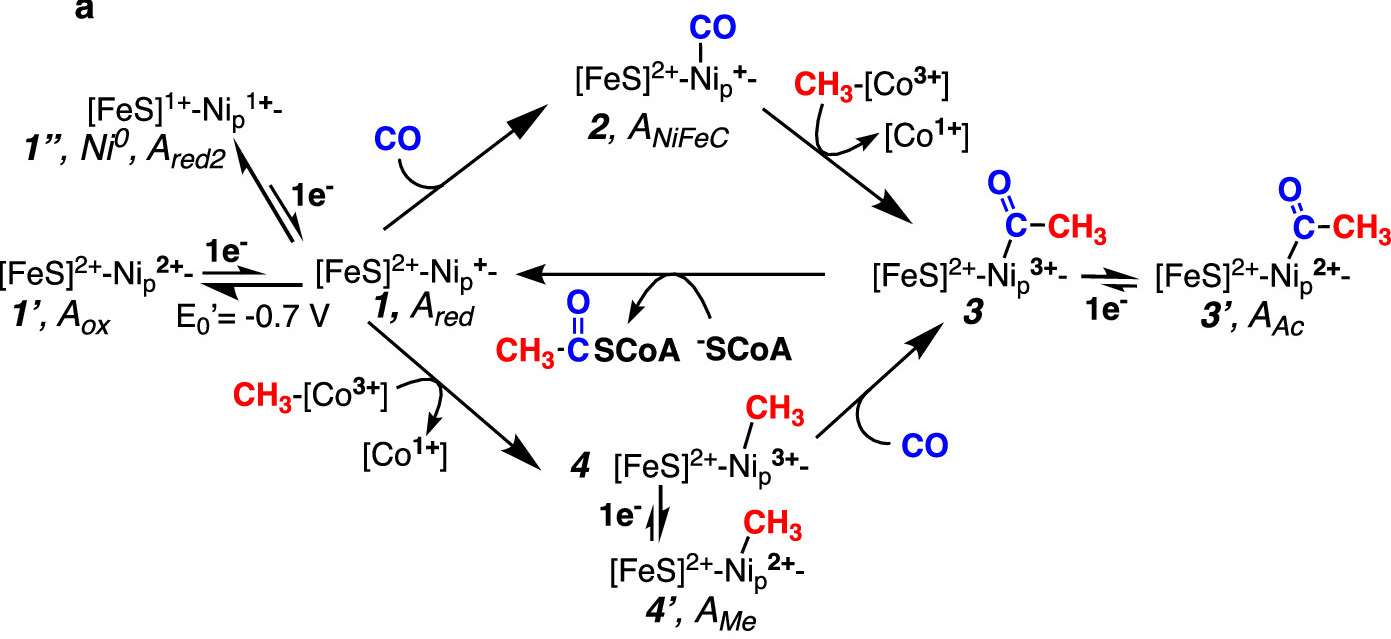
Proposed Acetyl-CoA Synthase Mechanism Including Electrochemical Coupling. The Wood-Ljungdahl catalytical cycle is shown with the addition of proposed intermediates and formal oxidation states in acetyl-CoA synthase.
[Reprinted under a Creative Commons license (CC BY-NC-ND 4.0) from Can et al. 2023. DOI:10.1021/jacs.3c01772]
The Science
The Wood-Ljungdahl pathway (WLP) is one of nature’s most elegant methods for chemically transforming carbon. The pathway is ancient, present in the last universal common ancestor to life on earth, and the only carbon fixation pathway known to produce more energy (net ATP) than it consumes. The WLP scrubs CO2 from the atmosphere, converting the carbon to energy and cellular building material. The process occurs within a large multi-unit protein complex comprising carbon monoxide dehydrogenase (CODH), which turns CO2 into CO, and acetyl-CoA synthase (ACS), which combines CO and a CH3– group donated by a corrinoid iron-sulfur protein (CFeSP) to produce acetate in the form of acetyl-CoA from the original CO2 molecule. Globally, anaerobes produce 1013 kg of acetate annually via the WLP. The pathway represents an ideal system upon which to develop biomimetic catalysts for atmospheric CO2 capture, but its underlying mechanism must first be understood.
Unique nickel (Ni)-based and iron-based active sites lie at the hearts of CODH and ACS where acetate and acetyl-CoA form through a series of Ni-based organometallic intermediates. The exact mechanism of acetate synthesis from CO and CH3 has been intensely studied for several decades, with two proposed mechanisms at the center of debate. Both propose that CO and CH3 reactants bind to a single nickel site (termed Nip), but each proposes a different oxidation state and electronic configuration for the reactant-bound Nip. In the paramagnetic mechanism, CO and CH3 react with Nip to form a series of paramagnetic Ni(I) and Ni(III) intermediates; in the other mechanism, catalytically active Nip species are formally diamagnetic Ni(0) and Ni(II).
In a collaborative study supported in part by the Structural Molecular Biology Resource at the SLAC National Accelerator Laboratory, researchers solved the geometric and electronic structures of the remaining uncharacterized organometallic intermediates of ACS using X-ray absorption spectroscopy (XAS) and other techniques. The structures revealed that ACS binds CO, CH3, and acetate through direct Ni-C bond formation, and that the Ni-CH3 intermediate is paramagnetic. In addition, the paramagnetic intermediates transformed under experimental conditions into their diamagnetic analogs and these diamagnetic species appeared to be recruited into the catalytic cycle as well.
To explain the existence of both catalytically relevant paramagnetic Ni(III) and diamagnetic Ni(II) species, the researchers proposed a novel electrochemical coupling mechanism. Under this mechanistic framework, Nip(I) (or Nip(I)-CO) reacts with methyl-Co(III) to generate Nip(III)-methyl (or Nip(III)-acetyl), which are reducible to their Nip(II) counterparts via interaction with excess reductant. These reduced intermediates are then recruited back into active catalysis through binding of the next substrate.
The Impact
This work details the remaining undescribed pieces of an ancient and critically important carbon fixation pathway using XAS and provides a new conceptual framework to bridge two previously competing mechanistic models of acetyl-CoA synthase (ACS).
Funding
This research was supported by a Department of Energy-Basic Energy Sciences Field Work Proposal 100593, a National Institutes of Health grant GM125924, a National Institutes of Health grant R35-GM141758, and a National Institutes of Health grant GM111097.The Stanford Synchrotron Radiation Lightsource is supported by the U.S. Department of Energy Office of Basic Energy Science under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the NIH National Institute of General Medical Sciences (including P41GM103393).
Related Links
References
Can, M., et al. 2023. “Characterization of Methyl- and Acetyl-Ni Intermediates in Acetyl CoA Synthase Formed during Anaerobic CO2 and CO Fixation,” J. Am. Chem. Soc. 145(25), 13696-13708. DOI:10.1021/jacs.3c01772
