Watching the Evolution of a Protein’s Function
12/09/2012
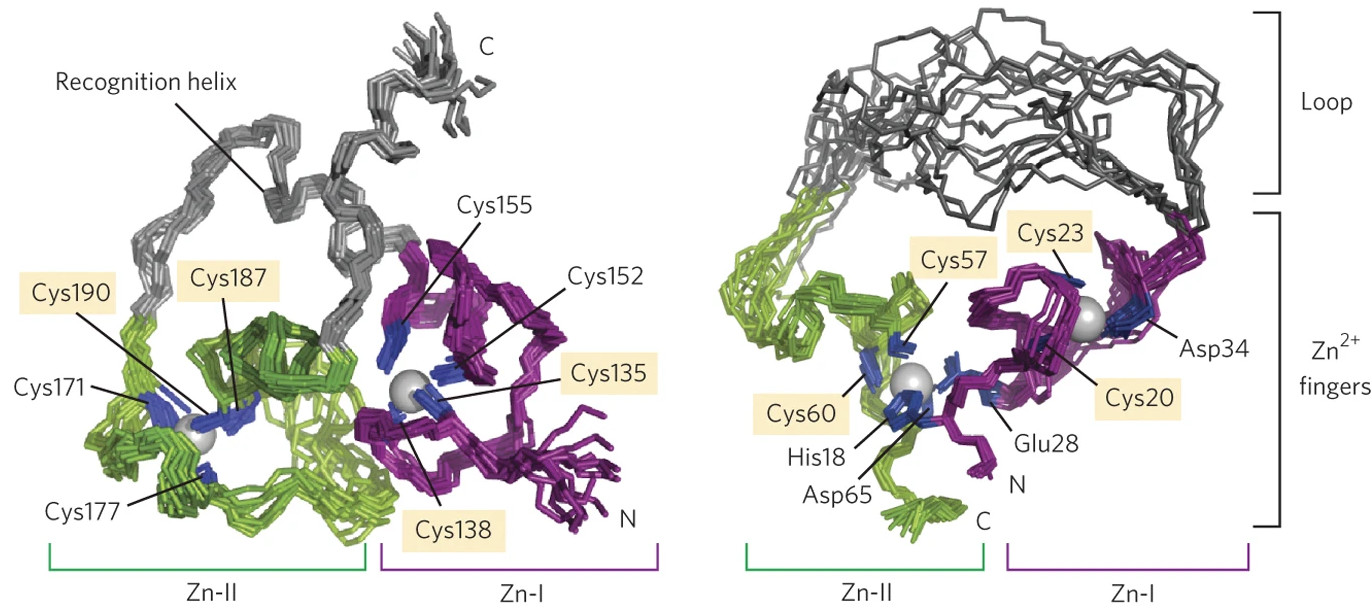
Comparison of the 3D structures of the noncatalytic protein hRXRα (left) and the ligase enzyme 10C (right) which was artificially evolved from it. Although both proteins contain two zinc fingers, the overall structures are substantially different. [Image reprinted by permission from Macmillan Publishers Ltd: Chao, F.A., et al. “Structure and dynamics of a primordial catalytic fold generated by in vitro evolution.” Nat. Chem. Biol. 9(2), 81–83. © 2013.]
The Science
A major challenge to large-scale biofuels production is developing enzymes that are highly efficient at converting biomass components into usable fuels.
The Impact
Using directed evolution—a process by which organisms or genes are artificially evolved to develop nucleic acid mutations or produce new proteins—researchers have determined the structural basis for converting a noncatalytic small protein into an effective enzyme for linking RNA molecules.
Summary
Enzymes are proteins structurally configured to catalyze conversions of substrates to products. Thousands of protein structures are known, including those of many valuable enzymes. However, much less is known about how small changes in a protein’s composition can alter its three-dimensional structure, control its catalytic efficiency, or even convert a protein with no catalytic function into an efficient catalyst. Scientists in this study used an extended X-ray absorption fine structure (EXAFS) station at the Stanford Synchrotron Radiation Lightsource to determine the active-site structure of a newly synthesized enzyme. The EXAFS experiments showed the exact chemical environment of each zinc atom in the new enzyme, leading to an explanation of why it developed the catalytic activity.
Funding Acknowledgements
Work supported by U.S. National Aeronautics and Space Administration (NASA) Agreement no. NNX09AH70A, through the NASA Astrobiology Institute–Ames Research Center (to F.-A.C., A.M., L.C. and B.S.); Minnesota Medical Foundation (to B.S.), and U.S. National Institutes of Health (NIH; T32 GM08347 to J.C.H., T32 DE007288 to L.R.M., GM100310 to G.V., and P41 RR001209). Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC National Accelerator Laboratory (SLAC) operations funded by the Office of Basic Energy Sciences (OBES), U.S. Department of Energy (DOE) Office of Science. SSRL Structural Molecular Biology program supported by NIH National Center for Research Resources (NCRR) and Office of Biological Environmental Research (OBER), DOE Office of Science.
Related Links
- BER Resource: Structural Molecular Biology Resource
- Feature Story: Watching the evolution of a protein’s function
References
F.-A. Chao, A. Morelli, J. C. Haugner, III, L. Churchfield, L. N. Hagmann, L. Shi, L. R. Masterson, R. Sarangi, G. Veglia and B. Seeling, “Structure and Dynamics of a Primordial Catalytic Fold Generated by in vitro Evolution,” Nat. Chem. Biol. 9, 81 (2013). DOI: 10.1038/nchembio.1138
