X-ray Footprinting Solves Mystery of Metal-Breathing Protein
08/14/2017
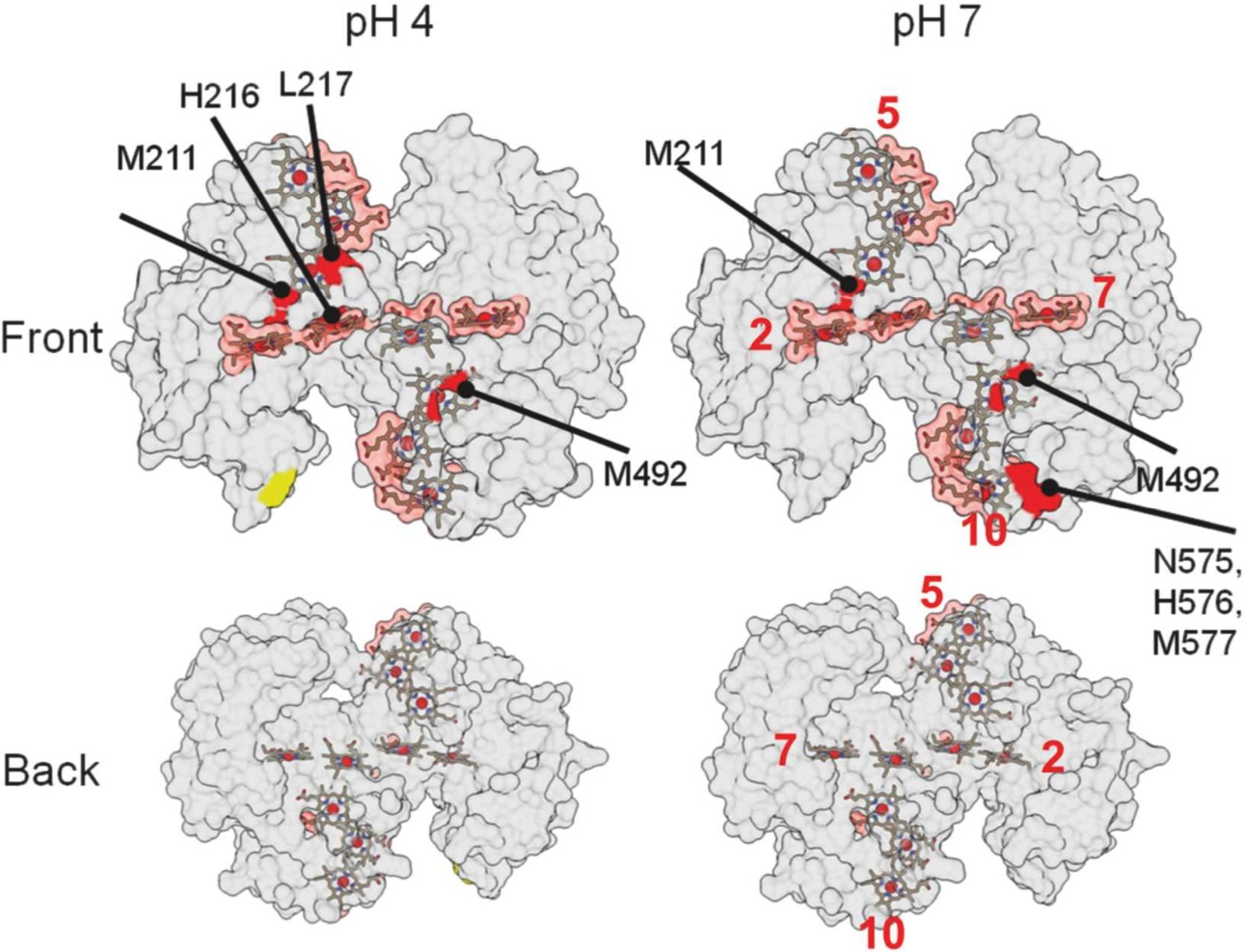
Results from X-ray footprinting mass spectrometry (XFMS) experiments at Lawrence Berkeley National Laboratory’s (LBNL) Advanced Light Source, mapped out on these three-dimensional structural renderings of a protein, helped researchers identify where the protein binds with a mineral. Red areas indicate possible binding areas. [Reprinted with permission from Fukushima, T., et al. DOI:10.1021/jacs.7b06560. Copyright (2017) American Chemical Society.]
The Summary
Scientists have discovered the details of an unconventional coupling between a protein from the metal-reducing bacterium Shewanella oneidensis and a mineral that allows the bacterium to breathe when oxygen is unavailable. The work could help answer a long-standing question in microbiology: how do bacterial proteins interact directly with minerals to transfer electrons and allow the microbe to live?
The researchers used an X-ray-based technique known as “footprinting” to pinpoint the chemical connections between the bacterial protein and nanoparticles composed of iron and oxygen. Footprinting reveals interactions of proteins in a near-native environment. The technique identified a surprisingly small and weak binding site that had already been mapped, but it also revealed how the site binds to metal-containing minerals—a feat that conventional techniques had been unable to accomplish.
The study provides insight into ways to design proteins with better electronic connections that can be used to build more sensitive bioelectronic sensors. It could also lead to new innovations in linking proteins to other materials for bio-based electronic devices, such as sensors that can diagnose disease or detect contaminants. And it could help researchers understand and control the chemical reactions sparked by these protein-material interactions and, eventually, how organisms remodel their environment and make biominerals.
Instruments and Facilities
X-ray footprinting was performed using X-ray beamline 5.3.1 at Lawrence Berkeley National Laboratory’s Advanced Light Source (ALS), followed by mass spectrometry at the Biological Nanostructures Facility at the Molecular Foundry.
Funding
Work was supported by the Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory (LBNL), performed at the Molecular Foundry and Advanced Light Source and used resources of the Joint BioEnergy Institute (JBEI), supported by the Office of Basic Energy Sciences (OBES) and Office of Biological and Environmental Research (OBER), U.S. Department of Energy (DOE) Office of Science.
Related Links
- X-ray Footprinting Solves Mystery of Metal-Breathing Protein
- Footprinting Technique Gives ALS Users New Insights
References
Fukushima, T., et al. “The Molecular Basis for Binding of an Electron Transfer Protein to a Metal Oxide Surface.” J. Am. Chem. Soc. 139(36), 12647–12654, (2017). [DOI:10.1021/jacs.7b06560]
