X-ray Spectroscopy Reveals Potassium-Bonding Environment
01/29/2024
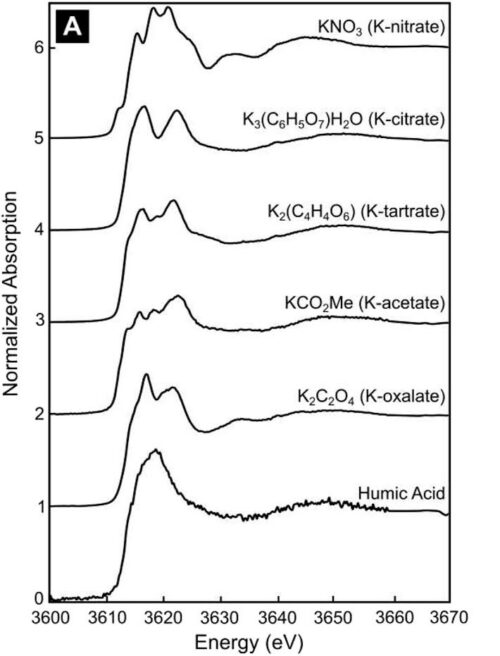
Potassium K-Edge XANES Spectroscopy of Various Organic Salts. The stacked spectra facilitate a comparison of the different signatures displayed by different organic salts bonded to potassium. With the exception of humic acid, the organic salts each show multiple unique spectral features (i.e., peaks) that can be used to identify the compounds in complex biological samples.
[Reprinted with permission from Richardson, J. A., et. al. “X-ray Absorption Spectroscopy and Theoretical Investigations of the Effect of Extended Ligands in Potassium Organic Matter Interaction.” The Journal of Chemical Physics 160(4). Copyright 2024, AIP Publishing. This article may be downloaded for personal use only. Any other use requires prior permission of the author and AIP Publishing.]
The Science
Potassium (K) is an essential element for plant growth. Soils can contain up to 3% K by weight, but the majority is structurally bound within minerals and considered non-bioavailable. Therefore, most environments are K-limited, even when supplied with fertilizer.
Weathering of K-rich minerals has the capacity to increase bioavailable K in the rhizosphere, but abiotic mineral weathering is too slow to support crop growth. Fortunately, several microbial species use mechanical and chemical processes to weather mineral surfaces and release critical nutrients to the local environment. However, the molecular mechanisms underpinning the biochemical processes involved in microbial nutrient sourcing from minerals are poorly understood.
To better understand how microbes source K from minerals, scientists at the Stanford Synchrotron Radiation Lightsource performed X-ray absorption spectroscopy (XAS) on K-organic salts, including acetate, citrate, nitrate, oxalate, and tartrate, which are frequently observed as acids secreted by soil microbes.
Results showed that XAS spectra are associated with extended organic salt ligands, and unique and distinct spectral features are associated with second-shell nitrogen compared to carbon. The improved understanding of K bonding environments with organic compounds provides an important toolkit to understand how K is transformed by microbial processes and made bioavailable for plant uptake.
The Impact
This research shows that the bonding environment of potassium in different salts of organic acids has different detectable signatures using XAS. The resulting spectra can inform whether potassium is associated with carbon, nitrogen, or oxygen. This will enable future research to fingerprint the type of organic compound bonded to K in complex biological and environmental samples; something that was not previously known to be possible. A further impact of this research is that characterization of these signatures will enable future studies to spatially distinguish among potassium organic molecules in natural soils.
The Summary
K-organic salts displayed feature-rich XAS spectra, each demonstrating numerous unique features spanning 13 eV, despite similar first shell bonding environments. To identify the electronic transitions that give rise to some of the unique spectral features in the organic salts, researchers used computational tools including molecular dynamics (MD), time-dependent density functional theory (TD-DFT), and full multiple scattering (FMS) in OCEAN, to simulate the experimental spectra.
Funding
A portion of this research was performed on a project award (10.46936/expl.proj.2021.60169/60008211) from the Environmental Molecular Sciences Laboratory, a DOE Office of Science User Facility, sponsored by the Biological and Environmental Research program under Contract No. DE-AC05-76RL01830. The use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, was supported by the U.S.
Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-76SF00515. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and the National Institutes of Health, National Institute of General Medical Sciences (Grant No. P30GM133894).
Related Links
- BER Resource: Structural Molecular Biology Resource
- BER Resource: Environmental Molecular Sciences Laboratory
- EMSL News: Unraveling How Potassium Bound to Soil Minerals is Made Bioavailable for Uptake by Plants
References
Richardson, J. A., Kim, H., Kas, J. J., You, X., Andersen, A., Ginovska, B., Bhattacharjee, A., and Sarangi, R. 2024. “X-ray Absorption Spectroscopy and Theoretical Investigations of the Effect of Extended Ligands in Potassium Organic Matter Interaction.” The Journal of Chemical Physics 160(4). DOI:10.1063/5.0183603.
