“Zip Code” Mechanism for Hormone Signaling Revealed
01/17/2018
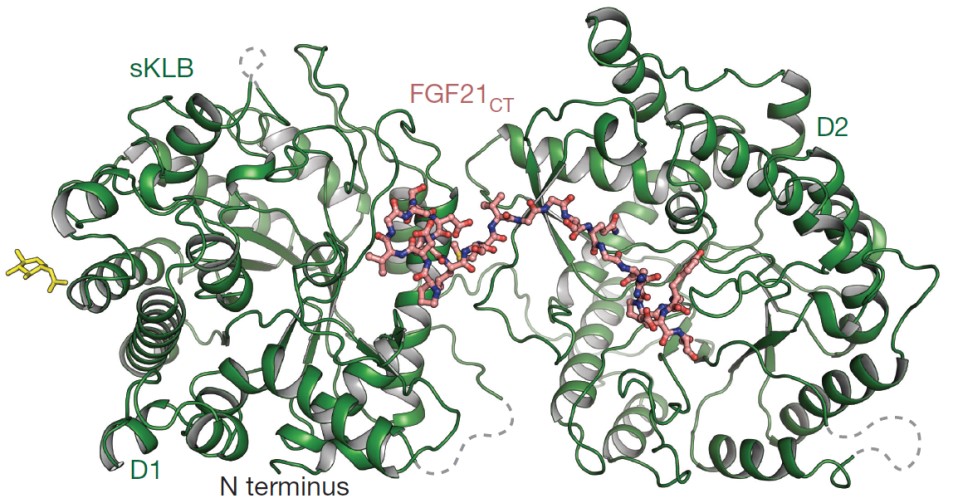
Crystal structure of multikinase inhibitor sKLB bound to fibroblast growth factor (FGF) FGF21CT reveals two distinct binding sites. Structure of extracellular domain of ß-Klotho “longevity” protein (green ribbons) bound to FGF21 hormone (salmon ball-and-stick) unveils critical molecular interactions required for hormone binding and cell activation. Yellow sticks denote nitrogen (N)-linked glycans. Grey dashed lines denote regions that do not exhibit significant electron density. About 30 angsroms (Å) apart, the FGF binding sites on the ß-Klotho D1 and D2 domains are located on the opposite side of the molecule from the flexible linker that connects the two glycosidase domains and may contribute to the interdomain dynamic properties that enable complex formation with ligands and FGF receptors (FGFRs). [Reprinted by permission from Springer Nature: Lee, S., et al. “Structures of ß-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signaling.” Nature 553, 501–505 (2018). DOI:10.1038/nature25010. Copyright 2018]
Named after the Greek goddess who spun the thread of life, Klotho proteins play an important role in the regulation of longevity and metabolism. Alpha-Klotho is a membrane-spanning protein expressed predominantly in the kidney, as well as in the brain. Mice lacking a-klotho exhibit a range of signs associated with aging and have elevated blood phosphate levels. Like a-klotho, ß-klotho functions as a co-receptor for endocrine fibroblast growth factors (FGFs). FGF21 is secreted from the liver following fasting, acting in fat cells and the brain to induce metabolic adaptation to fasting and responses to stress. Although FGF receptors (FGFRs) are expressed in a wide range of tissues, expression of the ß-klotho “longevity” protein in the liver, fat, and brain restricts the target organs of these endocrine FGFs.
In a recent Yale Medical School-led study, researchers revealed the three-dimensional (3D), high-resolution structure of ß-Klotho, illuminating its intricate mechanism and potential for antiaging therapeutics, as well as for treating a wide range of medical conditions. X-ray crystallography data revealed critical molecular interactions required for hormone binding and cell activation. The specific “zip code”-like interactions of ß-klotho receptors appear to regulate critical metabolic processes in the liver, kidneys, and brain, among other organs. Analysis yielded several insights, including that ß-Klotho is the primary receptor that binds to FGF21, a key hormone that stimulates insulin sensitivity and glucose metabolism, causing weight loss. The researchers believe this new understanding can guide the development of therapies by improving the biological activity of FGF21. Also found were a new variant of FGF21 that has 10 times higher potency and cellular activity and the evidence of how a structurally related enzyme (glycosidase) that breaks down sugars evolved into a receptor for a hormone that lowers blood sugar. Researchers believe the untangled ß-Klotho structure presents a platform for exploring and developing agents that either enhance or block the pathway, enabling therapies for conditions such as liver cancer and bone diseases.
Instruments and Facilities
X-ray data were collected from ß-klotho–receptor crystals at Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory, beamline 14-1 as part of the program to aid National Synchrotron Light Source (NSLS) users prior to NSLS-II operations.
Funding Acknowledgements
The National Synchrotron Lightsource (NSLS) and Stanford Synchrotron Radiation Lightsource (SSRL) were supported by P41GM111244, P41GM103393, DE-SC0012704, and DE-AC02-76SF00515. The researchers thank NE-CAT (P41 GM103403) and APS (DE-AC02-06CH11357). Research was also supported by the National Institutes of Health (NIH) grant 1S10OD018007 and NIH Award S10RR026992-0110. J. Steyaert received financial support from INSTRUCT (European Strategy Forum on Research Infrastructures [ESFRI], participate with or without funding [FWO]).
Related Links
- BER Resource: Center for BioMolecular Structure
- Feature Story: Secrets of longevity protein revealed in new study
- Science Highlight: Structures of β-klotho reveal a "zip code"-like mechanism for endocrine FGF signaling
References
Lee, S., et al. “Structures of ß-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signaling.” Nature 553, 501–505 (2018). [DOI:10.1038/nature25010]
